Lysyl oxidase-like 2 depletion is protective in age-associated vascular stiffening
- PMID: 31002285
- PMCID: PMC6692735
- DOI: 10.1152/ajpheart.00670.2018
Lysyl oxidase-like 2 depletion is protective in age-associated vascular stiffening
Abstract
Vascular stiffening and its sequelae are major causes of morbidity and mortality in the elderly. The increasingly accepted concept of "smooth muscle cell (SMC) stiffness syndrome" along with matrix deposition has emerged in vascular biology to account for the mechanical phenotype of arterial aging, but the molecular targets remain elusive. In this study, using an unbiased proteomic analysis, we identified lysyl oxidase-like 2 (LOXL2) as a critical SMC mediator for age-associated vascular stiffening. We tested the hypothesis that loss of LOXL2 function is protective in aging-associated vascular stiffening. We determined that exogenous and endogenous nitric oxide markedly decreased LOXL2 abundance and activity in the extracellular matrix of isolated SMCs and LOXL2 endothelial cells suppress LOXL2 abundance in the aorta. In a longitudinal study, LOXL2+/- mice were protected from age-associated increase in pulse-wave velocity, an index of vascular stiffening, as occurred in littermate wild-type mice. Using isolated aortic segments, we found that LOXL2 mediates vascular stiffening in aging by promoting SMC stiffness, augmented SMC contractility, and vascular matrix deposition. Together, these studies establish LOXL2 as a nodal point for a new therapeutic approach to treat age-associated vascular stiffening. NEW & NOTEWORTHY Increased central vascular stiffness augments risk of major adverse cardiovascular events. Despite significant advances in understanding the genetic and molecular underpinnings of vascular stiffening, targeted therapy has remained elusive. Here, we show that lysyl oxidase-like 2 (LOXL2) drives vascular stiffening during aging by promoting matrix remodeling and vascular smooth muscle cell stiffening. Reduced LOXL2 expression protects mice from age-associated vascular stiffening and delays the onset of isolated systolic hypertension, a major consequence of stiffening.
Keywords: aging; lysyl oxidase-like 2; pulse-wave velocity; vascular stiffness.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
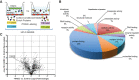


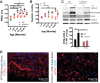

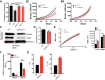
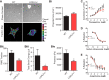
Comment in
-
Lysyl oxidases as driving forces behind age-related macrovascular rigidity.Am J Physiol Heart Circ Physiol. 2019 Jul 1;317(1):H37-H38. doi: 10.1152/ajpheart.00264.2019. Epub 2019 May 17. Am J Physiol Heart Circ Physiol. 2019. PMID: 31099611 Free PMC article. No abstract available.
References
-
- Bia D, Zócalo Y, Farro I, Torrado J, Farro F, Florio L, Olascoaga A, Brum J, Alallón W, Negreira C, Lluberas R, Armentano RL. Integrated evaluation of age-related changes in structural and functional vascular parameters used to assess arterial aging, subclinical atherosclerosis, and cardiovascular risk in uruguayan adults: CUiiDARTE project. Int J Hypertens 2011: 1–12, 2011. doi: 10.4061/2011/587303. - DOI - PMC - PubMed
-
- Bignon M, Pichol-Thievend C, Hardouin J, Malbouyres M, Bréchot N, Nasciutti L, Barret A, Teillon J, Guillon E, Etienne E, Caron M, Joubert-Caron R, Monnot C, Ruggiero F, Muller L, Germain S. Lysyl oxidase-like protein-2 regulates sprouting angiogenesis and type IV collagen assembly in the endothelial basement membrane. Blood 118: 3979–3989, 2011. doi: 10.1182/blood-2010-10-313296. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

