Vascular Endothelial Growth Factor Delivery to Placental Basal Plate Promotes Uterine Artery Remodeling in the Primate
- PMID: 31002314
- PMCID: PMC6542484
- DOI: 10.1210/en.2019-00059
Vascular Endothelial Growth Factor Delivery to Placental Basal Plate Promotes Uterine Artery Remodeling in the Primate
Abstract
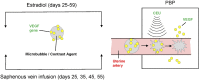
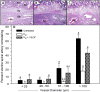
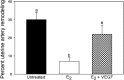
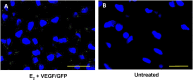
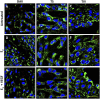
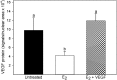
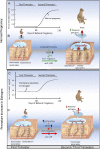
Extravillous trophoblast (EVT) uterine artery remodeling (UAR) promotes placental blood flow, but UAR regulation is unproven. Elevating estradiol (E2) in early baboon pregnancy suppressed UAR and EVT vascular endothelial growth factor (VEGF) expression, but this did not prove that VEGF mediated this process. Therefore, our primate model of prematurely elevating E2 and contrast-enhanced ultrasound cavitation of microbubble (MB) carriers was used to deliver VEGF DNA to the placental basal plate (PBP) to establish the role of VEGF in UAR. Baboons were treated on days 25 to 59 of gestation (term, 184 days) with E2 alone or with E2 plus VEGF DNA-conjugated MBs briefly infused via a maternal peripheral vein on days 25, 35, 45, and 55. At each of these times an ultrasound beam was directed to the PBP to collapse the MBs and release VEGF DNA. VEGF DNA-labeled MBs per contrast agent was localized in the PBP but not the fetus. Remodeling of uterine arteries >25 µm in diameter on day 60 was 75% lower (P < 0.001) in E2-treated (7% ± 2%) than in untreated baboons (30% ± 4%) and was restored to normal by E2/VEGF. VEGF protein levels (signals/nuclear area) within the PBP were twofold lower (P < 0.01) in E2-treated (4.2 ± 0.9) than in untreated (9.8 ± 2.8) baboons and restored to normal by E2/VEGF (11.9 ± 1.6), substantiating VEGF transfection. Thus, VEGF gene delivery selectively to the PBP prevented the decrease in UAR elicited by prematurely elevating E2 levels, establishing the role of VEGF in regulating UAR in vivo during primate pregnancy.
Copyright © 2019 Endocrine Society.
Figures

References
-
- Ramsey EM, Houston ML, Harris JW. Interactions of the trophoblast and maternal tissues in three closely related primate species. Am J Obstet Gynecol. 1976;124(6):647–652. - PubMed
-
- Pijnenborg R, Dixon G, Robertson WB, Brosens I. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta. 1980;1(1):3–19. - PubMed
-
- Enders AC, King BF. Early stages of trophoblastic invasion of the maternal vascular system during implantation in the macaque and baboon. Am J Anat. 1991;192(4):329–346. - PubMed
-
- Frank HG, Kaufmann P. Nonvillous parts and trophoblast invasion. In: Benirschke K, Kaufmann P, Baergen RN, eds. Pathology of the Human Placenta. 5th ed. New York, NY: Springer; 2006:191–312.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

