Factors Associated With Use of Sipuleucel-T to Treat Patients With Advanced Prostate Cancer
- PMID: 31002323
- PMCID: PMC6481456
- DOI: 10.1001/jamanetworkopen.2019.2589
Factors Associated With Use of Sipuleucel-T to Treat Patients With Advanced Prostate Cancer
Abstract
Importance: Sipuleucel-T is an immunotherapy that has been approved for use in patients with asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer (mCRPC). However, sipuleucel-T may not be available to some patients because of logistics, cost, and practice structure.
Objective: To identify factors associated with the adoption of sipuleucel-T across the United States.
Design, setting, and participants: In this retrospective cohort study, patients with prostate cancer who received therapy for mCRPC (docetaxel, abiraterone acetate, enzalutamide, cabazitaxel, radium 223, or sipuleucel-T) from January 1, 2010, through June 30, 2016, were identified in a large claims database of commercially insured patients. Patients who received sipuleucel-T were compared with patients who received any of the other treatments for mCRPC but did not receive sipuleucel-T. Data were analyzed from May 3, 2018, to February 24, 2019.
Exposures: Sipuleucel-T treatment.
Main outcomes and measures: Patterns of treatment that involved the use of sipuleucel-T were elucidated, and binomial logistic regression was conducted to determine patient and physician factors that were associated with the use of sipuleucel-T and whether patients received sipuleucel-T in isolation or concurrently with other therapies.
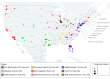
Results: Among 7272 patients who received a treatment for mCRPC, 730 (10.0%) received sipuleucel-T. Mean (SD) age of patients in the entire cohort was 73.2 (9.2) years; 6739 (92.7%) were non-Hispanic and 975 (13.4%) were black. In multivariable analysis, patients who were Hispanic (odds ratio [OR], 0.57; 95% CI, 0.38-0.86) or lived in the Pacific region (OR, 0.66; 95% CI, 0.45-0.97) had lower odds of receiving sipuleucel-T than patients who were not Hispanic or who lived in the South Atlantic region. Patients with higher incomes had greater odds of receiving sipuleucel-T than patients with incomes of less than $50 000 (OR, 1.29 [95% CI, 1.04-1.61] for $50 000-$99 000; OR, 1.43 [95% CI, 1.10-1.85] for >$99 000). Patients treated by a urologist had greater odds of receiving sipuleucel-T than patients not treated by a urologist (OR, 8.89; 95% CI, 7.10-11.11). Sixty-seven patients received concurrent therapies with sipuleucel-T, most commonly abiraterone or enzalutamide, but no factors were independently associated with patients receiving sipuleucel-T concurrent with other therapies for mCRPC.
Conclusions and relevance: In this study, 1 of 10 patients with prostate cancer who were treated for mCRPC received sipuleucel-T, with several variables associated with its use. Identifying disparities in receipt of sipuleucel-T may affect future access to this and other highly specialized cancer therapies by defining barriers to treatment that could be addressed in future studies.
Conflict of interest statement
Figures


References
-
- de Bono JS, Oudard S, Ozguroglu M, et al. ; TROPIC Investigators . Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147-1154. doi: 10.1016/S0140-6736(10)61389-X - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

