Effects of ginsenoside Rg3 on epigenetic modification in ovarian cancer cells
- PMID: 31002353
- PMCID: PMC6489025
- DOI: 10.3892/or.2019.7115
Effects of ginsenoside Rg3 on epigenetic modification in ovarian cancer cells
Abstract
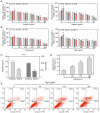
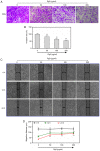
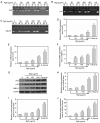
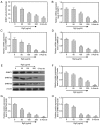
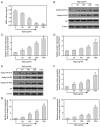
Epigenetic modifications are closely related to oncogene activation and tumor suppressor gene inactivation. The aim of this study was to determine the effects of ginsenoside Rg3 on epigenetic modification in ovarian cancer cells. Cell proliferation, metastasis, invasion and apoptosis were respectively determined using Cell Counting Kit‑8 (CCK‑8), wound healing, Transwell and flow cytometric assays. Methylation levels were determined using methylation specific PCR (MSP). Related‑factor expression was detected by conducting real‑time‑qPCR (RT‑qPCR) and western blotting. The results revealed that cell proliferation was inhibited by ginsenoside Rg3 (0, 25, 50, 100 and 200 µg/ml) in a time‑dependent manner (12, 24 and 48 h). Ginsenoside Rg3 (50, 100 and 200 µg/ml) was selected to treat cells in various experiments. When ovarian cells were treated with ginsenoside Rg3, cell apoptosis was observed to be promoted, while cell metastasis and invasion were inhibited at 48 h. The results of the present study revealed that in the promoter regions of p53, p16 and hMLH1, the methylation levels decreased, while the mRNA and protein levels significantly increased. The activities of DNMTs and mRNA as well as protein levels of DNMT1, DNMT3a and DNMT3b were decreased by Rg3. The data also demonstrated that the mRNA and protein levels of acetyl‑H3 K14/K9 and acetyl‑H4 K12/K5/K16 were increased by Rg3. Hence, ginsenoside Rg3 inhibited ovarian cancer cell viability, migration and invasion as well as promoted cell apoptosis.
Figures
References
-
- Henderson JT, Webber EM, Sawaya GF. U.S. preventive services task force evidence syntheses formerly systematic evidence reviews Screening for ovarian cancer: An updated evidence review for the U.S. preventive services task force. Agency for Healthcare Research and Quality (US) 2018 Report No.: 17-05231-EF-1. - PubMed
-
- Smith RA, Manassaram-Baptiste D, Brooks D, Doroshenk M, Fedewa S, Saslow D, Brawley OW, Wender R. Cancer screening in the United States, 2015: A review of current American cancer society guidelines and current issues in cancer screening. CA Cancer J Clin. 2015;65:30–54. doi: 10.3322/caac.21261. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

