Regulation of the unfolded protein response in yeast by oxidative stress
- PMID: 31002390
- PMCID: PMC6538422
- DOI: 10.1002/1873-3468.13389
Regulation of the unfolded protein response in yeast by oxidative stress
Abstract
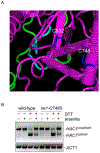
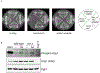
In the unfolded protein response (UPR), Ire1 activates Hac1 to coordinate the transcription of hundreds of genes to mitigate ER stress. Recent work in Caenorhabditis elegans suggests that oxidative stress inhibits this canonical Ire1 signalling pathway, activating instead an antioxidant stress response. We sought to determine whether this novel mode of UPR function also existed in yeast, where Ire1 has been best characterized. We show that the yeast UPR is also subject to inhibition by oxidative stress. Inhibition is mediated by a single evolutionarily conserved cysteine, and affects both luminal and membrane pathways of Ire1 activation. In yeast, Ire1 appears dispensable for resistance to oxidative stress and, therefore, the physiological significance of this pathway remains to be demonstrated.
Keywords: Ire1; arsenic; cysteine; oxidative stress; unfolded protein response.
© 2019 Federation of European Biochemical Societies.
Conflict of interest statement
Competing Interests
The authors declare no competing interests.
Figures


References
-
- Cox JS, Walter P. A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell. 1996. November 1;87(3):391–404. - PubMed
-
- Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 2000. April 28;101(3):249–58. - PubMed
-
- Oikawa D, Kimata Y, Kohno K, Iwawaki T. Activation of mammalian IRE1alpha upon ER stress depends on dissociation of BiP rather than on direct interaction with unfolded proteins. Exp Cell Res. 2009. September 10;315(15):2496–504. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

