Simulating Time-Dependent Patterns of Nonadherence by Patients With Breast Cancer to Adjuvant Oral Endocrine Therapy
- PMID: 31002563
- PMCID: PMC6873985
- DOI: 10.1200/CCI.18.00091
Simulating Time-Dependent Patterns of Nonadherence by Patients With Breast Cancer to Adjuvant Oral Endocrine Therapy
Abstract
Purpose: Nearly 40% of patients with breast cancer discontinue their adjuvant oral endocrine treatment (ET). We measured discontinuation rates of ET at a comprehensive cancer center. We then used an iterative approach to model patterns of determinants associated with discontinuation of ET.
Methods: Patients with nonmetastatic breast cancer receiving active adjuvant ET were approached by nurse practitioners to complete an anonymous survey at one time point. We simulated a prospective model by iteratively regressing adverse effects onto adherence status across windowed time periods of 2 to 3 consecutive years, bootstrapping the smaller group of nonadherent patients and subsampling the larger adherent group.
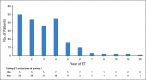
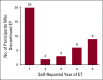
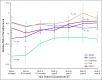
Results: From February to April 2013, 216 participants were enrolled in the study. Forty patients (18.5%) reported that they had discontinued ET during the first 5 years of ET, and an additional four patients (1.9%) missed > 20% of their doses. Using two-sided significance tests, simulations showed that all 13 ET adverse effects and reasons for discontinuation were significantly related to discontinuation at some time point during ET. Worry about ET cost (odds ratio [OR], 1.79), emotional distress (OR, 1.72), and bone and joint pain (OR, 1.69) were the three most impactful reasons for discontinuation, with varying patterns of influence over time.
Conclusion: These analyses provide preliminary evidence that there are varying patterns of discontinuation of ET. Although some reasons for discontinuation exerted a steady influence over the 6-year ET trajectory (ie, bone and joint pain), other reasons, such as cost, cognitive complaints, and general dislike of pills, became more important in the later years of ET.
Conflict of interest statement
Eileen H. Shinn
Stacy Moulder
William Fraser Symmans
No other potential conflicts of interest were reported.
Figures
References
-
- Moore S. Nonadherence in patients with breast cancer receiving oral therapies. Clin J Oncol Nurs. 2010;14:41–47. - PubMed
-
- Hadji P. Improving compliance and persistence to adjuvant tamoxifen and aromatase inhibitor therapy. Crit Rev Oncol Hematol. 2010;73:156–166. - PubMed
-
- Ma AM, Barone J, Wallis AE, et al. Noncompliance with adjuvant radiation, chemotherapy, or hormonal therapy in breast cancer patients. Am J Surg. 2008;196:500–504. - PubMed
-
- Yood MU, Owusu C, Buist DS, et al. Mortality impact of less-than-standard therapy in older breast cancer patients. J Am Coll Surg. 2008;206:66–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

