Randomized Trial of Intravenous Versus Intraperitoneal Chemotherapy Plus Bevacizumab in Advanced Ovarian Carcinoma: An NRG Oncology/Gynecologic Oncology Group Study
- PMID: 31002578
- PMCID: PMC6544459
- DOI: 10.1200/JCO.18.01568
Randomized Trial of Intravenous Versus Intraperitoneal Chemotherapy Plus Bevacizumab in Advanced Ovarian Carcinoma: An NRG Oncology/Gynecologic Oncology Group Study
Erratum in
-
Erratum.J Clin Oncol. 2019 Sep 1;37(25):2299. doi: 10.1200/JCO.19.01365. J Clin Oncol. 2019. PMID: 31461635 Free PMC article. No abstract available.
Abstract
Purpose: To evaluate the impact of two different intraperitoneal (IP) chemotherapy regimens on progression-free survival (PFS) among women with newly diagnosed advanced ovarian carcinoma.
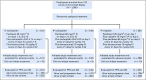
Methods: Eligible patients were randomly assigned to six cycles of IV paclitaxel 80 mg/m2 once per week with intravenous (IV) carboplatin area under the curve 6 (IV carboplatin) versus IV paclitaxel 80 mg/m2 once per week with IP carboplatin area under the curve 6 (IP carboplatin) versus once every 3 weeks IV paclitaxel 135 mg/m2 over 3 hours day 1, IP cisplatin 75 mg/m2 day 2, and IP paclitaxel 60 mg/m2 day 8 (IP cisplatin). All participants received bevacizumab 15 mg/kg IV every 3 weeks in cycles 2 to 22.
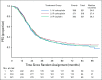
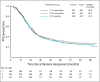
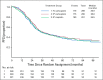
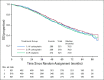
Results: A total of 1,560 participants were enrolled and had 84.8 months of follow-up. The median PFS duration was 24.9 months in the IV carboplatin arm, 27.4 months in the IP carboplatin arm, and 26.2 months in the IP cisplatin arm. For the subgroup of 1,380 patients with stage II/III and residual disease of 1 cm or less, median PFS was 26.9 (IV-carboplatin), 28.7 (IP-carboplatin), and 27.8 months (IP cisplatin), respectively. Median PFS for patients with stage II/III and no residual disease was 35.9, 38.8, and 35.5 months, respectively. Median overall survival for all enrolled was 75.5, 78.9, and 72.9 months, respectively, and median overall survival for stage II/III with no gross residual disease was 98.8 months, 104.8 months, and not reached. Mean patient-reported Functional Assessment of Cancer Therapy neurotoxicity scores (Gynecologic Oncology Group) were similar for all arms, but the mean Trial Outcome Index of the Functional Assessment of Cancer Therapy-Ovary scores during chemotherapy were statistically worse in the IP cisplatin arm.
Conclusion: Compared with the IV carboplatin reference arm, the duration of PFS was not significantly increased with either IP regimen when combined with bevacizumab and was better tolerated than IP cisplatin.
Trial registration: ClinicalTrials.gov NCT00951496.
Figures
Comment in
-
Advanced Epithelial Ovarian Cancer: Do More Options Mean Greater Benefits?J Clin Oncol. 2019 Jun 1;37(16):1359-1364. doi: 10.1200/JCO.19.00500. Epub 2019 Apr 19. J Clin Oncol. 2019. PMID: 31002579
-
Intraperitoneal Chemotherapy in Advanced Ovarian Cancer: Old and Novel Questions.J Clin Oncol. 2019 Nov 20;37(33):3168-3169. doi: 10.1200/JCO.19.01163. Epub 2019 Sep 25. J Clin Oncol. 2019. PMID: 31553677 No abstract available.
-
Reply to F. Tomao et al and E.A. De Jaeghere et al.J Clin Oncol. 2019 Nov 20;37(33):3170. doi: 10.1200/JCO.19.02091. Epub 2019 Sep 25. J Clin Oncol. 2019. PMID: 31553678 No abstract available.
-
Treatment of Ovarian Cancer With Intraperitoneal Platinum: Heating Up the Debate.J Clin Oncol. 2019 Nov 20;37(33):3169-3170. doi: 10.1200/JCO.19.01179. Epub 2019 Sep 25. J Clin Oncol. 2019. PMID: 31553679 No abstract available.
References
-
- Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. - PubMed
-
- Landrum LM, Gold MA, Moore KN, et al. Intraperitoneal chemotherapy for patients with advanced epithelial ovarian cancer: A review of complications and completion rates. Gynecol Oncol. 2008;108:342–347. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 CA180868/CA/NCI NIH HHS/United States
- UG1 CA233324/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- U10 CA180798/CA/NCI NIH HHS/United States
- UG1 CA233193/CA/NCI NIH HHS/United States
- UG1 CA233191/CA/NCI NIH HHS/United States
- P30 CA016058/CA/NCI NIH HHS/United States
- U10 CA180833/CA/NCI NIH HHS/United States
- UG1 CA233290/CA/NCI NIH HHS/United States
- UG1 CA189867/CA/NCI NIH HHS/United States
- UG1 CA233331/CA/NCI NIH HHS/United States
- U10 CA180822/CA/NCI NIH HHS/United States
- UG1 CA233328/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

