A transgenic zebrafish line for in vivo visualisation of neutrophil myeloperoxidase
- PMID: 31002727
- PMCID: PMC6474608
- DOI: 10.1371/journal.pone.0215592
A transgenic zebrafish line for in vivo visualisation of neutrophil myeloperoxidase
Abstract
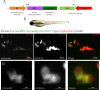
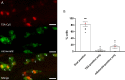
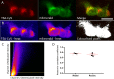
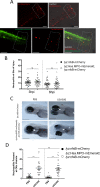
The neutrophil enzyme myeloperoxidase (MPO) is a major enzyme made by neutrophils to generate antimicrobial and immunomodulatory compounds, notably hypochlorous acid (HOCl), amplifying their capacity for destroying pathogens and regulating inflammation. Despite its roles in innate immunity, the importance of MPO in preventing infection is unclear, as individuals with MPO deficiency are asymptomatic with the exception of an increased risk of candidiasis. Dysregulation of MPO activity is also linked with inflammatory conditions such as atherosclerosis, emphasising a need to understand the roles of the enzyme in greater detail. Consequently, new tools for investigating granular dynamics in vivo can provide useful insights into how MPO localises within neutrophils, aiding understanding of its role in preventing and exacerbating disease. The zebrafish is a powerful model for investigating the immune system in vivo, as it is genetically tractable, and optically transparent. To visualise MPO activity within zebrafish neutrophils, we created a genetic construct that expresses human MPO as a fusion protein with a C-terminal fluorescent tag, driven by the neutrophil-specific promoter lyz. After introducing the construct into the zebrafish genome by Tol2 transgenesis, we established the Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496 line, and confirmed transgene expression in zebrafish neutrophils. We observed localisation of MPO-mEmerald within a subcellular location resembling neutrophil granules, mirroring MPO in human neutrophils. In Spotless (mpxNL144) larvae-which express a non-functional zebrafish myeloperoxidase-the MPO-mEmerald transgene does not disrupt neutrophil migration to sites of infection or inflammation, suggesting that it is a suitable line for the study of neutrophil granule function. We present a new transgenic line that can be used to investigate neutrophil granule dynamics in vivo without disrupting neutrophil behaviour, with potential applications in studying processing and maturation of MPO during development.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89: 3503–21. Available: http://www.ncbi.nlm.nih.gov/pubmed/9160655 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

