Long-term biochemical progression-free survival following brachytherapy for prostate cancer: Further insight into the role of short-term androgen deprivation and intermediate risk group subclassification
- PMID: 31002732
- PMCID: PMC6474628
- DOI: 10.1371/journal.pone.0215582
Long-term biochemical progression-free survival following brachytherapy for prostate cancer: Further insight into the role of short-term androgen deprivation and intermediate risk group subclassification
Abstract
Introduction: Brachytherapy is a well-established treatment of localized prostate cancer. Few studies have documented long-term results, specifically biochemical progression-free survival (bPFS) in men with brachytherapy alone, with or without short-term androgen deprivation therapy (ADT), or in combination with external beam radiotherapy (EBRT). Our aim was to analyze long-term bPFS of brachytherapy treated patients.
Materials and methods: Retrospective analysis of 1457 patients with low and intermediate risk prostate cancer treated with brachytherapy alone (1255) or combined with EBRT (202). Six-months ADT was administrated for all EBRT combined patients and for prostate volume downsizing when >55 cc (328). Failure was by the Phoenix definition. Kaplan-Meier analysis and multivariate Cox regression estimated and compared 10-yr and 15-yr rates of bPFS.
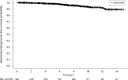
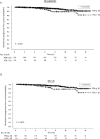
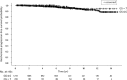
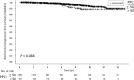
Results: Median follow-up was 6.1 yr. Ten and 15-yr bPFS rates of the entire cohort were 93.2% and 89.2%, respectively. On multivariate analysis, PSA density (PSAD), ADT and clinical stage were significantly associated with failure. The most powerful independent factor was PSAD with a HR of 3.5 (95% CI, 1.7-7.4) for PSAD above 0.15. No significant difference was found between low and intermediate risks patients regardless of treatment regimen. However, comparison of two intermediate risk groups, Gleason score (GS) 7, PSA<20 ng/ml versus GS≤6 and PSA = 10-20 ng/ml, revealed 10- and 15-yr bPFS rates of 94.2% and 94.2% compared to 88.2% and 79.9%, (P = 0.022), respectively. ADT improved bPFS rates in low risk patients. The ten and 15-yr bPFS rates were 97.6% and 94.6% compared to 92.3% and 88.2%, (P = 0.020), respectively.
Conclusions: Our retrospective large scale study suggests that brachytherapy provides excellent long-term bPFS rates in low and intermediate risk disease. Combination of brachytherapy with EBRT yields favorable outcomes in GS 7 intermediate risk patients and short-term ADT has a positive effect on outcomes in low risk patients. Further prospective studies are warranted to discriminate the role of adding either EBRT and/or ADT to brachytherapy protocols.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
American Brachytherapy Society Task Group Report: Use of androgen deprivation therapy with prostate brachytherapy-A systematic literature review.Brachytherapy. 2017 Mar-Apr;16(2):245-265. doi: 10.1016/j.brachy.2016.11.017. Epub 2017 Jan 16. Brachytherapy. 2017. PMID: 28110898 Free PMC article. Review.
-
Do androgen deprivation and the biologically equivalent dose matter in low-dose-rate brachytherapy for intermediate-risk prostate cancer?Cancer Med. 2016 Sep;5(9):2314-22. doi: 10.1002/cam4.820. Epub 2016 Jul 25. Cancer Med. 2016. PMID: 27456710 Free PMC article.
-
The need for androgen deprivation therapy in patients with intermediate-risk prostate cancer treated with dose-escalated external beam radiation therapy.Can J Urol. 2017 Feb;24(1):8656-8662. Can J Urol. 2017. PMID: 28263132
-
High-risk prostate cancer with Gleason score 8-10 and PSA level ≤15 ng/mL treated with permanent interstitial brachytherapy.Int J Radiat Oncol Biol Phys. 2011 Nov 15;81(4):992-6. doi: 10.1016/j.ijrobp.2010.07.006. Epub 2010 Oct 6. Int J Radiat Oncol Biol Phys. 2011. PMID: 20932674
-
Benefits and Risks of Primary Treatments for High-risk Localized and Locally Advanced Prostate Cancer: An International Multidisciplinary Systematic Review.Eur Urol. 2020 May;77(5):614-627. doi: 10.1016/j.eururo.2020.01.033. Epub 2020 Mar 4. Eur Urol. 2020. PMID: 32146018
Cited by
-
Virtual HDR Boost for Prostate Cancer: Rebooting a Classic Treatment Using Modern Tech.Cancers (Basel). 2023 Mar 28;15(7):2018. doi: 10.3390/cancers15072018. Cancers (Basel). 2023. PMID: 37046680 Free PMC article. Review.
-
Comparison of EBRT and I-125 seed brachytherapy concerning outcome in intermediate-risk prostate cancer.Strahlenther Onkol. 2021 Nov;197(11):986-992. doi: 10.1007/s00066-021-01815-z. Epub 2021 Aug 5. Strahlenther Onkol. 2021. PMID: 34351453 Free PMC article.
-
Retrospective Analysis of Clinical Outcomes of Stereotactic Body Radiation Therapy for Localized Prostate Cancer at an Asian Cancer Specialist Centre.Asian Pac J Cancer Prev. 2023 Feb 1;24(2):545-550. doi: 10.31557/APJCP.2023.24.2.545. Asian Pac J Cancer Prev. 2023. PMID: 36853303 Free PMC article.
References
-
- Bruner DW, Moughan J, Prestidge BR, Sanda MG, Bice W, Michalski J, Ibbott G, Amin M, Catton C, Donavanik V, Gay H, Brachman D, Frank SJ, Papagikos M, Rosenthal SA, Matulonis U, Sadeghi A, Winter K SH. Patient Reported Outcomes of NRG Oncology/RTOG 0232: A Phase III Study Comparing Combined External Beam Radiation and Transperineal Interstitial Permanent Brachytherapy with Brachytherapy Alone in Intermediate Risk Prostate Cancer. Abstr Present Annu Meet Am Soc Radiat Oncol (ASTRO) San Antonio, TX. 2018;
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

