Structure and mechanism of TagA, a novel membrane-associated glycosyltransferase that produces wall teichoic acids in pathogenic bacteria
- PMID: 31002736
- PMCID: PMC6493773
- DOI: 10.1371/journal.ppat.1007723
Structure and mechanism of TagA, a novel membrane-associated glycosyltransferase that produces wall teichoic acids in pathogenic bacteria
Abstract
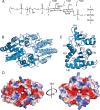
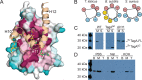
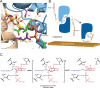
Staphylococcus aureus and other bacterial pathogens affix wall teichoic acids (WTAs) to their surface. These highly abundant anionic glycopolymers have critical functions in bacterial physiology and their susceptibility to β-lactam antibiotics. The membrane-associated TagA glycosyltransferase (GT) catalyzes the first-committed step in WTA biosynthesis and is a founding member of the WecB/TagA/CpsF GT family, more than 6,000 enzymes that synthesize a range of extracellular polysaccharides through a poorly understood mechanism. Crystal structures of TagA from T. italicus in its apo- and UDP-bound states reveal a novel GT fold, and coupled with biochemical and cellular data define the mechanism of catalysis. We propose that enzyme activity is regulated by interactions with the bilayer, which trigger a structural change that facilitates proper active site formation and recognition of the enzyme's lipid-linked substrate. These findings inform upon the molecular basis of WecB/TagA/CpsF activity and could guide the development of new anti-microbial drugs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Brown S, Xia G, Luhachack LG, Campbell J, Meredith TC, Chen C, et al. Methicillin resistance in Staphylococcus aureus requires glycosylated wall teichoic acids. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(46):18909–14. 10.1073/pnas.1209126109 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

