Biogenesis, quality control, and structural dynamics of proteins as explored in living cells via site-directed photocrosslinking
- PMID: 31002747
- PMCID: PMC6566533
- DOI: 10.1002/pro.3627
Biogenesis, quality control, and structural dynamics of proteins as explored in living cells via site-directed photocrosslinking
Abstract
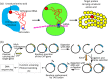
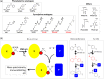
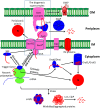
Protein biogenesis and quality control are essential to maintaining a functional pool of proteins and involve numerous protein factors that dynamically and transiently interact with each other and with the substrate proteins in living cells. Conventional methods are hardly effective for studying dynamic, transient, and weak protein-protein interactions that occur in cells. Herein, we review how the site-directed photocrosslinking approach, which relies on the genetic incorporation of a photoreactive unnatural amino acid into a protein of interest at selected individual amino acid residue positions and the covalent trapping of the interacting proteins upon ultraviolent irradiation, has become a highly efficient way to explore the aspects of protein contacts in living cells. For example, in the past decade, this approach has allowed the profiling of the in vivo substrate proteins of chaperones or proteases under both physiologically optimal and stressful (e.g., acidic) conditions, mapping residues located at protein interfaces, identifying new protein factors involved in the biogenesis of membrane proteins, trapping transiently formed protein complexes, and snapshotting different structural states of a protein. We anticipate that the site-directed photocrosslinking approach will play a fundamental role in dissecting the detailed mechanisms of protein biogenesis, quality control, and dynamics in the future.
Keywords: dynamics of proteins; in vivo protein photocrosslinking; membrane protein biogenesis; molecular chaperones; proteases; protein quality control.
© 2019 The Protein Society.
Figures

References
-
- Blobel G (2000) Protein targeting. ChemBioChem 1:86–102. - PubMed
-
- Chang Z. Biogenesis of secretory proteins: folding and quality control in the endoplasmic reticulum In: Bradshawa RA, Stahl P, Eds, 2016, Encyclopedia of cell biology. Waltham, MA: Academic Press; p. 535–544.
-
- Balchin D, Hayer‐Hartl M, Hartl FU (2016) In vivo aspects of protein folding and quality control. Science 353:aac4354. - PubMed
-
- Sontag EM, Samant RS, Frydman J (2017) Mechanisms and functions of spatial protein quality control. Annu Rev Biochem 86:97–122. - PubMed
-
- Chiti F, Dobson CM (2017) Protein misfolding, amyloid formation, and human disease: a summary of progress over the last decade. Annu Rev Biochem 86:27–68. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

