Curcumin and Intestinal Inflammatory Diseases: Molecular Mechanisms of Protection
- PMID: 31003422
- PMCID: PMC6514688
- DOI: 10.3390/ijms20081912
Curcumin and Intestinal Inflammatory Diseases: Molecular Mechanisms of Protection
Abstract
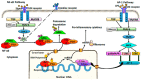
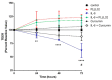
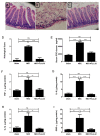
Intestinal inflammatory diseases, such as Crohn's disease, ulcerative colitis, and necrotizing enterocolitis, are becoming increasingly prevalent. While knowledge of the pathogenesis of these related diseases is currently incomplete, each of these conditions is thought to involve a dysfunctional, or overstated, host immunological response to both bacteria and dietary antigens, resulting in unchecked intestinal inflammation and, often, alterations in the intestinal microbiome. This inflammation can result in an impaired intestinal barrier allowing for bacterial translocation, potentially resulting in systemic inflammation and, in severe cases, sepsis. Chronic inflammation of this nature, in the case of inflammatory bowel disease, can even spur cancer growth in the longer-term. Recent research has indicated certain natural products with anti-inflammatory properties, such as curcumin, can help tame the inflammation involved in intestinal inflammatory diseases, thus improving intestinal barrier function, and potentially, clinical outcomes. In this review, we explore the potential therapeutic properties of curcumin on intestinal inflammatory diseases, including its antimicrobial and immunomodulatory properties, as well as its potential to alter the intestinal microbiome. Curcumin may play a significant role in intestinal inflammatory disease treatment in the future, particularly as an adjuvant therapy.
Keywords: Crohn’s disease; curcumin; inflammatory bowel disease; necrotizing enterocolitis; ulcerative colitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Dias A.M., Correia A., Pereira M.S., Almeida C.R., Alves I., Pinto V., Catarino T.A., Mendes N., Leander M., Oliva-Teles M.T., et al. Metabolic control of T cell immune response through glycans in inflammatory bowel disease. Proc. Natl. Acad. Sci. USA. 2018;115:E4651–E4660. doi: 10.1073/pnas.1720409115. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

