Synthesis of New Derivatives of Benzofuran as Potential Anticancer Agents
- PMID: 31003438
- PMCID: PMC6514909
- DOI: 10.3390/molecules24081529
Synthesis of New Derivatives of Benzofuran as Potential Anticancer Agents
Abstract
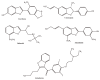
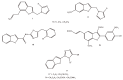
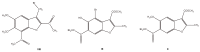
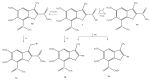
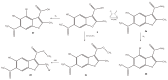
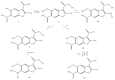
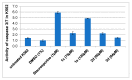
The results of our previous research indicated that some derivatives of benzofurans, particularly halogeno-derivatives, are selectively toxic towards human leukemia cells. Continuing our work with this group of compounds we here report new data on the synthesis as well as regarding the physico-chemical and biological characterization of fourteen new derivatives of benzofurans, including six brominated compounds. The structures of all new compounds were established by spectroscopic methods (1H- and, 13C-NMR, ESI MS), and elemental analyses. Their cytotoxicity was evaluated against K562 (leukemia), MOLT-4 (leukemia), HeLa (cervix carcinoma), and normal cells (HUVEC). Five compounds (1c, 1e, 2d, 3a, 3d) showed significant cytotoxic activity against all tested cell lines and selectivity for cancer cell lines. The SAR analysis (structure-activity relationship analysis) indicated that the presence of bromine introduced to a methyl or acetyl group that was attached to the benzofuran system increased their cytotoxicity both in normal and cancer cells.
Keywords: HeLa; K562; MOLT-4; benzofurans; chemical synthesis; cytotoxic properties.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
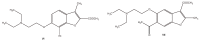
Similar articles
-
Synthesis and cytotoxic properties of halogen and aryl-/heteroarylpiperazinyl derivatives of benzofurans.Anticancer Agents Med Chem. 2015;15(1):115-21. doi: 10.2174/187152061501141204124709. Anticancer Agents Med Chem. 2015. PMID: 25482722
-
Design, synthesis and cytotoxic activity evaluation of new aminosubstituted benzofurans.Med Chem. 2014;10(6):619-27. doi: 10.2174/15734064113096660049. Med Chem. 2014. PMID: 24047215
-
Synthesis and cytotoxic evaluation of usnic acid benzylidene derivatives as potential anticancer agents.Nat Prod Res. 2021 Apr;35(7):1097-1106. doi: 10.1080/14786419.2019.1639176. Epub 2019 Jul 13. Nat Prod Res. 2021. PMID: 31303058
-
Potential of cyclopenta[b]benzofurans from Aglaia species in cancer chemotherapy.Anticancer Agents Med Chem. 2006 Jul;6(4):319-45. doi: 10.2174/187152006777698123. Anticancer Agents Med Chem. 2006. PMID: 16842234 Review.
-
An update on benzofuran inhibitors: a patent review.Expert Opin Ther Pat. 2019 Nov;29(11):841-870. doi: 10.1080/13543776.2019.1673727. Epub 2019 Oct 14. Expert Opin Ther Pat. 2019. PMID: 31560232 Review.
Cited by
-
Microwave assisted synthesis of 2-amino-4-chloro-pyrimidine derivatives: Anticancer and computational study on potential inhibitory action against COVID-19.Arab J Chem. 2022 Dec;15(12):104366. doi: 10.1016/j.arabjc.2022.104366. Epub 2022 Oct 19. Arab J Chem. 2022. PMID: 36276298 Free PMC article.
-
Ultrasonic-Assisted Synthesis of Benzofuran Appended Oxadiazole Molecules as Tyrosinase Inhibitors: Mechanistic Approach through Enzyme Inhibition, Molecular Docking, Chemoinformatics, ADMET and Drug-Likeness Studies.Int J Mol Sci. 2022 Sep 19;23(18):10979. doi: 10.3390/ijms231810979. Int J Mol Sci. 2022. PMID: 36142889 Free PMC article.
-
Oxygen- and Sulphur-Containing Heterocyclic Compounds as Potential Anticancer Agents.Appl Biochem Biotechnol. 2022 Dec;194(12):6438-6467. doi: 10.1007/s12010-022-04099-w. Epub 2022 Jul 28. Appl Biochem Biotechnol. 2022. PMID: 35900713 Review.
-
Exploring the Synergistic Anticancer Potential of Benzofuran-Oxadiazoles and Triazoles: Improved Ultrasound- and Microwave-Assisted Synthesis, Molecular Docking, Hemolytic, Thrombolytic and Anticancer Evaluation of Furan-Based Molecules.Molecules. 2022 Feb 2;27(3):1023. doi: 10.3390/molecules27031023. Molecules. 2022. PMID: 35164286 Free PMC article.
-
Rational synthesis, anticancer activity, and molecular docking studies of novel benzofuran liked thiazole hybrids.Mol Divers. 2023 Jun;27(3):1345-1357. doi: 10.1007/s11030-022-10493-7. Epub 2022 Jul 19. Mol Divers. 2023. PMID: 35852708
References
-
- Masubuchi M., Kawasaki K., Ebiike H., Ikeda Y., Tsujii S., Sogabe S., Fujii T., Sakata K., Shiratori Y., Aoki Y., et al. Design and synthesis of novel benzofurans as new class of antifungal agents targeting fungal N-myristoyltransferase. Part 1. Bioorg. Med. Chem. Lett. 2001;11:1833–1837. doi: 10.1016/S0960-894X(01)00319-5. - DOI - PubMed
-
- Wróbel J.E., Dietrich A.J., Antane M.M. Benzotiophenes, Benzofurans, and Indoles useful in the treatment of insulin resistance and hyperglycemia. 6,251,936. U.S. Patent. 2001 Jun 26;:1–57.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

