Targeting Wnt Signaling via Notch in Intestinal Carcinogenesis
- PMID: 31003440
- PMCID: PMC6520938
- DOI: 10.3390/cancers11040555
Targeting Wnt Signaling via Notch in Intestinal Carcinogenesis
Abstract
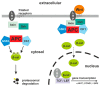
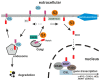
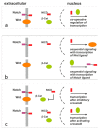
Proliferation and differentiation of intestinal epithelial cells is assisted by highly specialized and well-regulated signaling cascades. The Wnt pathway, which is one of the fundamental pathways in the intestine, contributes to the organization of proliferative intestinal crypts by positioning and cycling of intestinal stem cells and their derivatives. The Wnt pathway promotes differentiation of intestinal secretory cell types along the crypt-plateau and crypt-villus axis. In contrast to the Wnt pathway, the intestinal Notch cascade participates in cellular differentiation and directs progenitor cells towards an absorptive fate with diminished numbers of Paneth and goblet cells. Opposing activities of Notch and Wnt signaling in the regulation of intestinal stem cells and the enterocytic cell fate have been elucidated recently. In fact, targeting Notch was able to overcome tumorigenesis of intestinal adenomas, prevented carcinogenesis, and counteracted Paneth cell death in the absence of caspase 8. At present, pharmacological Notch inhibition is considered as an interesting tool targeting the intrinsic Wnt pathway activities in intestinal non-neoplastic disease and carcinogenesis.
Keywords: Notch; Wnt; cancer; caspase 8; intestine; lncRNA; targeting.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

