Recent Advances on Metal-Free, Visible-Light- Induced Catalysis for Assembling Nitrogen- and Oxygen-Based Heterocyclic Scaffolds
- PMID: 31003464
- PMCID: PMC6515354
- DOI: 10.3390/molecules24081533
Recent Advances on Metal-Free, Visible-Light- Induced Catalysis for Assembling Nitrogen- and Oxygen-Based Heterocyclic Scaffolds
Abstract
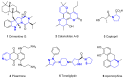
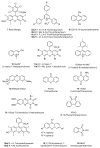
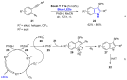
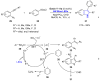
Heterocycles are important class of structures, which occupy a major space in the domain of natural and bioactive compounds. For this reason, development of new synthetic strategies for their controllable synthesis became of special interests. The development of novel photoredox systems with wide-range application in organic synthesis is particularly interesting. Organic dyes have been widely applied as photoredox catalysts in organic synthesis. Their low costs compared to the typical photocatalysts based on transition metals make them an excellent alternative. This review describes proceedings since 2015 in the area of application of metal-free, visible-light-mediated catalysis for assembling various heterocyclic scaffolds containing five- and six-membered rings bearing nitrogen and oxygen heteroatoms.
Keywords: heterocycles; organic dyes; photocatalysis; photoredox; photoredox cyclization; visible-light-induced catalysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

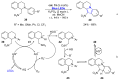
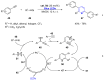
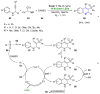
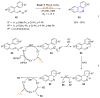
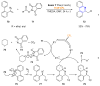
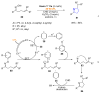
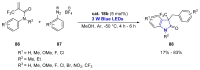
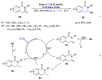
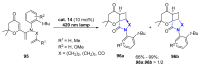
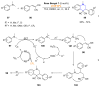
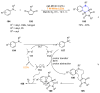
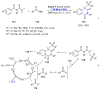
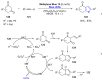
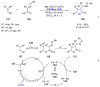
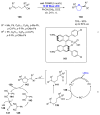
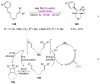
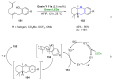
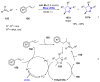
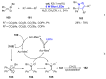




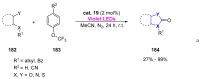
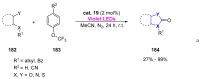


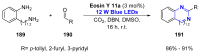
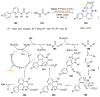
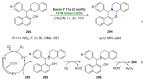
References
-
- Joule J.A., Mills K. Heterocyclic Chemistry. 5th ed. Wiley-Blackwell; Hoboken, NJ, USA: 2010.
-
- Pozharskii A.F., Soldatenkov A.T., Katritzky A.R. Heterocycles in Life and Society: An Introduction to Heterocyclic Chemistry, Biochemistry and Applications. 2nd ed. Wiley; Hoboken, NJ, USA: 2011.
-
- Eicher T., Hauptmann S., Speicher A. The Chemistry of Heterocycles: Structures, Reactions, Synthesis, and Applications. Wiley-VCH; Weinheim, Germany: 2013. 3rd Completely Revised and Enlarged ed.
-
- Creagh T., Ruckle J.L., Tolbert D.T., Giltner J., Eiznhamer D.A., Dutta B., Flavin M.T., Xu Z.Q. Safety and pharmacokinetics of single doses of (+)-Calanolide a, a novel, naturally occurring nonnucleoside reverse transcriptase inhibitor, in healthy, human immunodeficiency virus-negative human subjects. Antimicrob. Agents Chemother. 2001;45:1379–1386. doi: 10.1128/AAC.45.5.1379-1386.2001. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

