PMN-MDSCs Enhance CTC Metastatic Properties through Reciprocal Interactions via ROS/Notch/Nodal Signaling
- PMID: 31003475
- PMCID: PMC6514876
- DOI: 10.3390/ijms20081916
PMN-MDSCs Enhance CTC Metastatic Properties through Reciprocal Interactions via ROS/Notch/Nodal Signaling
Abstract
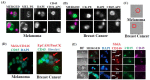
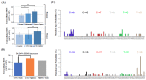
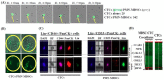
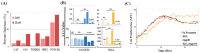
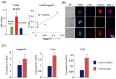
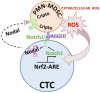
Intratumoral infiltration of myeloid-derived suppressor cells (MDSCs) is known to promote neoplastic growth by inhibiting the tumoricidal activity of T cells. However, direct interactions between patient-derived MDSCs and circulating tumors cells (CTCs) within the microenvironment of blood remain unexplored. Dissecting interplays between CTCs and circulatory MDSCs by heterotypic CTC/MDSC clustering is critical as a key mechanism to promote CTC survival and sustain the metastatic process. We characterized CTCs and polymorphonuclear-MDSCs (PMN-MDSCs) isolated in parallel from peripheral blood of metastatic melanoma and breast cancer patients by multi-parametric flow cytometry. Transplantation of both cell populations in the systemic circulation of mice revealed significantly enhanced dissemination and metastasis in mice co-injected with CTCs and PMN-MDSCs compared to mice injected with CTCs or MDSCs alone. Notably, CTC/PMN-MDSC clusters were detected in vitro and in vivo either in patients' blood or by longitudinal monitoring of blood from animals. This was coupled with in vitro co-culturing of cell populations, demonstrating that CTCs formed physical clusters with PMN-MDSCs; and induced their pro-tumorigenic differentiation through paracrine Nodal signaling, augmenting the production of reactive oxygen species (ROS) by PMN-MDSCs. These findings were validated by detecting significantly higher Nodal and ROS levels in blood of cancer patients in the presence of naïve, heterotypic CTC/PMN-MDSC clusters. Augmented PMN-MDSC ROS upregulated Notch1 receptor expression in CTCs through the ROS-NRF2-ARE axis, thus priming CTCs to respond to ligand-mediated (Jagged1) Notch activation. Jagged1-expressing PMN-MDSCs contributed to enhanced Notch activation in CTCs by engagement of Notch1 receptor. The reciprocity of CTC/PMN-MDSC bi-directional paracrine interactions and signaling was functionally validated in inhibitor-based analyses, demonstrating that combined Nodal and ROS inhibition abrogated CTC/PMN-MDSC interactions and led to a reduction of CTC survival and proliferation. This study provides seminal evidence showing that PMN-MDSCs, additive to their immuno-suppressive roles, directly interact with CTCs and promote their dissemination and metastatic potency. Targeting CTC/PMN-MDSC heterotypic clusters and associated crosstalks can therefore represent a novel therapeutic avenue for limiting hematogenous spread of metastatic disease.
Keywords: Heterotypic CTC clusters; biomarkers and signaling pathways; breast cancer; circulating tumor cells (CTCs); melanoma; mutual CTC/PMN-MDSC activation cycle; polymorphonuclear-myeloid derived suppressor cells (PMN-MDSCs).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Visualization and quantification of in vivo homing kinetics of myeloid-derived suppressor cells in primary and metastatic cancer.Theranostics. 2019 Aug 12;9(20):5869-5885. doi: 10.7150/thno.33275. eCollection 2019. Theranostics. 2019. PMID: 31534525 Free PMC article.
-
Epithelial-to-mesenchymal Transition Heterogeneity of Circulating Tumor Cells and Their Correlation With MDSCs and Tregs in HER2-negative Metastatic Breast Cancer Patients.Anticancer Res. 2021 Feb;41(2):661-670. doi: 10.21873/anticanres.14817. Anticancer Res. 2021. PMID: 33517270
-
Expansion of PMN-myeloid derived suppressor cells and their clinical relevance in patients with oral squamous cell carcinoma.Oral Oncol. 2019 Aug;95:157-163. doi: 10.1016/j.oraloncology.2019.06.004. Epub 2019 Jun 22. Oral Oncol. 2019. PMID: 31345384
-
Relevance of CTC Clusters in Breast Cancer Metastasis.Adv Exp Med Biol. 2020;1220:93-115. doi: 10.1007/978-3-030-35805-1_7. Adv Exp Med Biol. 2020. PMID: 32304082 Review.
-
Multi-omic features and clustering phenotypes of circulating tumor cells associated with metastasis and clinical outcomes.Int Rev Cell Mol Biol. 2025;392:67-100. doi: 10.1016/bs.ircmb.2024.03.009. Epub 2024 May 2. Int Rev Cell Mol Biol. 2025. PMID: 40287221 Review.
Cited by
-
An Immunological Perspective of Circulating Tumor Cells as Diagnostic Biomarkers and Therapeutic Targets.Life (Basel). 2022 Feb 21;12(2):323. doi: 10.3390/life12020323. Life (Basel). 2022. PMID: 35207611 Free PMC article. Review.
-
Single-Cell RNA Sequencing Uncovers Heterogeneous Circulating Tumor Cell Subsets in Breast Cancer.Cancers (Basel). 2022 Mar 4;14(5):1314. doi: 10.3390/cancers14051314. Cancers (Basel). 2022. PMID: 35267622 Free PMC article.
-
Remodeling the tumor microenvironment to overcome treatment resistance in HPV-negative head and neck cancer.Cancer Drug Resist. 2023 May 30;6(2):291-313. doi: 10.20517/cdr.2022.141. eCollection 2023. Cancer Drug Resist. 2023. PMID: 37457128 Free PMC article. Review.
-
The identification of a TNBC liver metastasis gene signature by sequential CTC-xenograft modeling.Mol Oncol. 2019 Sep;13(9):1913-1926. doi: 10.1002/1878-0261.12533. Epub 2019 Jun 19. Mol Oncol. 2019. PMID: 31216110 Free PMC article.
-
The Engagement Between MDSCs and Metastases: Partners in Crime.Front Oncol. 2020 Feb 18;10:165. doi: 10.3389/fonc.2020.00165. eCollection 2020. Front Oncol. 2020. PMID: 32133298 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

