Quantitative Analysis of Nuclear Lamins Imaged by Super-Resolution Light Microscopy
- PMID: 31003483
- PMCID: PMC6524165
- DOI: 10.3390/cells8040361
Quantitative Analysis of Nuclear Lamins Imaged by Super-Resolution Light Microscopy
Abstract
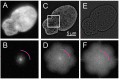
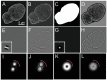
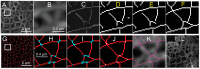
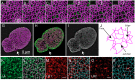
The nuclear lamina consists of a dense fibrous meshwork of nuclear lamins, Type V intermediate filaments, and is ~14 nm thick according to recent cryo-electron tomography studies. Recent advances in light microscopy have extended the resolution to a scale allowing for the fine structure of the lamina to be imaged in the context of the whole nucleus. We review quantitative approaches to analyze the imaging data of the nuclear lamina as acquired by structured illumination microscopy (SIM) and single molecule localization microscopy (SMLM), as well as the requisite cell preparation techniques. In particular, we discuss the application of steerable filters and graph-based methods to segment the structure of the four mammalian lamin isoforms (A, C, B1, and B2) and extract quantitative information.
Keywords: computational geometry; delaunay triangulation; lamins; single molecule localization microscopy; steerable filters; structured illumination microscopy; voronoi tessellation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

