Preparation of Zirconium-89 Solutions for Radiopharmaceutical Purposes: Interrelation Between Formulation, Radiochemical Purity, Stability and Biodistribution
- PMID: 31003494
- PMCID: PMC6514948
- DOI: 10.3390/molecules24081534
Preparation of Zirconium-89 Solutions for Radiopharmaceutical Purposes: Interrelation Between Formulation, Radiochemical Purity, Stability and Biodistribution
Abstract
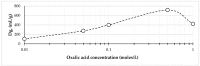
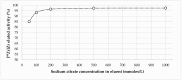
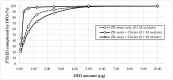
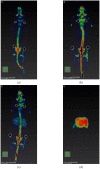
Zirconium-89 is a promising radionuclide for nuclear medicine. The aim of the present work was to find a suitable method for obtaining zirconium-89 solutions for radiopharmaceutical purposes. For this purpose, the ion exchange behavior of zirconium-89 solutions was studied. Radio-TLC (thin layer chromatography) and biodistribution studies were carried out to understand speciation of zirconium-89 complexes and their role in the development of new radiopharmaceuticals. Three methods of zirconium-89 isolation were studied using ZR (hydroxamate) and Chelex-100 resins. It was found that ZR-resin alone is not enough to obtain stable zirconium-89 formulations. An easy and effective method of reconstitution of [89Zr]Zr-oxalate to [89Zr]Zr-citrate using Chelex-100 resin was developed. Developed procedures allow obtaining [89Zr]Zr-oxalate (in 0.1 M sodium oxalate solution) and [89Zr]Zr-citrate (in 0.1-1.0 M sodium citrate solution). These solutions are perfectly suitable and convenient for radiopharmaceutical purposes. Our results prove [89Zr]Zr-citrate to be advantageous over [89Zr]Zr-oxalate. During evaluation of speciation of zirconium-89 complexes, a new TLC method was developed, since it was proved that there is no comprehensive method for analysis or zirconium-89 preparations. The new method provides valuable insights about the content of "active" ionic form of zirconium-89. The interrelation of the chromatographic behavior of zirconium-89 preparations and their biodistribution was studied.
Keywords: Chelex-100; DFO; ZR hydroxamate resin; citrate; oxalate; purification; radiochemical purity; stability; transchelation; zirconium-89.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures

References
-
- Knapp F.F., Dash A. Radiopharmaceuticals for Therapy. Springer; New Delhi, India: 2016. Introduction: Radiopharmaceuticals Play an Important Role in Both Diagnostic and Therapeutic Nuclear Medicine; pp. 3–23.
-
- Qin Z. Recent advances of injectable radiopharmaceuticals for nuclear imaging and therapy: A new era in nuclear medicine. Mater. Technol. 2015;30:B250–B255. doi: 10.1080/10667857.2015.1104829. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

