Enhanced Intestinal Permeability and Plasma Concentration of Metformin in Rats by the Repeated Administration of Red Ginseng Extract
- PMID: 31003498
- PMCID: PMC6523382
- DOI: 10.3390/pharmaceutics11040189
Enhanced Intestinal Permeability and Plasma Concentration of Metformin in Rats by the Repeated Administration of Red Ginseng Extract
Abstract
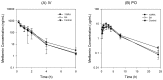
We aimed to assess the potential herb-drug interactions between Korean red ginseng extract (RGE) and metformin in rats in terms of the modulation of metformin transporters, such as organic cation transporter (Oct), multiple toxin and extrusion protein (Mate), and plasma membrane monoamine transporter (Pmat). Single treatment of RGE did not inhibit the in vitro transport activity of OCT1/2 up to 500 µg/mL and inhibited MATE1/2-K with high IC50 value (more than 147.8 µg/mL), suggesting that concomitant used of RGE did not directly inhibit OCT- and MATE-mediated metformin uptake. However, 1-week repeated administration of RGE (1.5 g/kg/day) (1WRA) to rats showed different alterations in mRNA levels of Oct1 depending on the tissue type. RGE increased intestinal Oct1 but decreased hepatic Oct1. However, neither renal Oct1/Oct2 nor Mate1/Pmat expression in duodenum, jejunum, ileum, liver, and kidney were changed in 1WRA rats. RGE repeated dose also increased the intestinal permeability of metformin; however, the permeability of 3-O-methyl-d-glucose and Lucifer yellow was not changed in 1WRA rats, suggesting that the increased permeability of metformin by multiple doses of RGE is substrate-specific. On pharmacokinetic analysis, plasma metformin concentrations following intravenous injection were not changed in 1WRA, consistent with no significant change in renal Oct1, Oct2, and mate1. Repeated doses of RGE for 1 week significantly increased the plasma concentration of metformin, with increased half-life and urinary excretion of metformin following oral administration of metformin (50 mg/kg), which could be attributed to the increased absorption of metformin. In conclusion, repeated administration of RGE showed in vivo pharmacokinetic herb-drug interaction with metformin, with regard to its plasma exposure and increased absorption in rats. These results were consistent with increased intestinal Oct1 and its functional consequence, therefore, the combined therapeutic efficacy needs further evaluation before the combination and repeated administration of RGE and metformin, an Oct1 substrate drug.
Keywords: herb–drug interaction; intestinal permeability; metformin; organic cation transporter; red ginseng extract (RGE).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Herb-Drug Interaction of Red Ginseng Extract and Ginsenoside Rc with Valsartan in Rats.Molecules. 2020 Jan 31;25(3):622. doi: 10.3390/molecules25030622. Molecules. 2020. PMID: 32023909 Free PMC article.
-
Organic cation transporter and multidrug and toxin extrusion 1 co-mediated interaction between metformin and berberine.Eur J Pharm Sci. 2019 Jan 15;127:282-290. doi: 10.1016/j.ejps.2018.11.010. Epub 2018 Nov 11. Eur J Pharm Sci. 2019. PMID: 30428337
-
Mechanistic in vitro studies confirm that inhibition of the renal apical efflux transporter multidrug and toxin extrusion (MATE) 1, and not altered absorption, underlies the increased metformin exposure observed in clinical interactions with cimetidine, trimethoprim or pyrimethamine.Pharmacol Res Perspect. 2017 Oct;5(5):e00357. doi: 10.1002/prp2.357. Pharmacol Res Perspect. 2017. PMID: 28971610 Free PMC article.
-
Polyspecific organic cation transporters and their impact on drug intracellular levels and pharmacodynamics.Pharmacol Res. 2016 Sep;111:237-246. doi: 10.1016/j.phrs.2016.06.002. Epub 2016 Jun 16. Pharmacol Res. 2016. PMID: 27317943 Free PMC article. Review.
-
Transport of Drugs and Endogenous Compounds Mediated by Human OCT1: Studies in Single- and Double-Transfected Cell Models.Front Pharmacol. 2021 Apr 22;12:662535. doi: 10.3389/fphar.2021.662535. eCollection 2021. Front Pharmacol. 2021. PMID: 33967805 Free PMC article. Review.
Cited by
-
Inhibitory Effect of AB-PINACA, Indazole Carboxamide Synthetic Cannabinoid, on Human Major Drug-Metabolizing Enzymes and Transporters.Pharmaceutics. 2020 Oct 29;12(11):1036. doi: 10.3390/pharmaceutics12111036. Pharmaceutics. 2020. PMID: 33138123 Free PMC article.
-
Concomitant Administration of Red Ginseng Extract with Lactic Acid Bacteria Increases the Plasma Concentration of Deglycosylated Ginsenosides in Healthy Human Subjects.Biomolecules. 2022 Dec 17;12(12):1896. doi: 10.3390/biom12121896. Biomolecules. 2022. PMID: 36551324 Free PMC article.
-
Simultaneous Analysis of a Combination of Anti-Hypertensive Drugs, Fimasartan, Amlodipine, and Hydrochlorothiazide, in Rats Using LC-MS/MS and Subsequent Application to Pharmacokinetic Drug Interaction with Red Ginseng Extract.Toxics. 2022 Sep 30;10(10):576. doi: 10.3390/toxics10100576. Toxics. 2022. PMID: 36287856 Free PMC article.
-
Herb-Drug Interaction of Red Ginseng Extract and Ginsenoside Rc with Valsartan in Rats.Molecules. 2020 Jan 31;25(3):622. doi: 10.3390/molecules25030622. Molecules. 2020. PMID: 32023909 Free PMC article.
-
Bile duct ligation differently regulates protein expressions of organic cation transporters in intestine, liver and kidney of rats through activation of farnesoid X receptor by cholate and bilirubin.Acta Pharm Sin B. 2023 Jan;13(1):227-245. doi: 10.1016/j.apsb.2022.06.010. Epub 2022 Jun 16. Acta Pharm Sin B. 2023. PMID: 36815051 Free PMC article.
References
LinkOut - more resources
Full Text Sources

