Preparation and Physicochemical Stability of Liquid Oral Dosage Forms Free of Potentially Harmful Excipient Designed for Pediatric Patients
- PMID: 31003500
- PMCID: PMC6523203
- DOI: 10.3390/pharmaceutics11040190
Preparation and Physicochemical Stability of Liquid Oral Dosage Forms Free of Potentially Harmful Excipient Designed for Pediatric Patients
Abstract
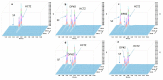
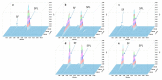
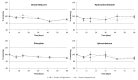
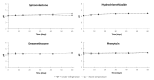
Dexamethasone, hydrochlorothiazide, spironolactone, and phenytoin are commonly used in neonates, but no age-appropriate formulation containing these active pharmaceutical ingredients (APIs) is commercially available. Thus, pharmaceutical compounding of the liquid oral dosage form is required to enable newborn administration. A problem common to the compounded preparations described in the literature is that they include potentially harmful excipients (PHEs). Therefore, the aim of this study was to evaluate the feasibility of compounding oral liquid dosage forms free of PHE, containing dexamethasone, hydrochlorothiazide, phenytoin, or spironolactone and to assess their physicochemical stability. Due to the poor water solubility of the targeted APIs, oral suspensions were compounded using Syrspend® SF-PH4 Dry, a suspending vehicle free of PHE. Four HPLC coupled to UV spectrometry (HPLC-UV) stability-indicating methods were developed and validated according to international guidelines to assay the strength of the targeted APIs. Whatever storage condition was used (5 ± 3 °C or 22 ± 4 °C), no significant degradation of API occurred in compounded oral suspensions. Overall, the results attest to the physical and chemical stability of the four oral liquid dosage forms over 60 days under regular storage temperatures. Finally, the use of the proposed oral suspensions provides a reliable solution to reduce the exposure of children to potentially harmful excipients.
Keywords: compounded preparation; liquid oral dosage form; pediatric patients; physicochemical stability; potentially harmful excipient.
Conflict of interest statement
The authors report no conflict of interest.
Figures


Similar articles
-
Compatibility of caffeine, carvedilol, clomipramine hydrochloride, folic acid, hydrochlorothiazide, loperamide hydrochloride, methotrexate, nadolol, naltrexone hydrochloride and pentoxifylline in SyrSpend SF PH4 oral suspensions.Eur J Hosp Pharm. 2016 Nov;23(6):352-358. doi: 10.1136/ejhpharm-2016-000903. Epub 2016 Mar 24. Eur J Hosp Pharm. 2016. PMID: 31156882 Free PMC article.
-
Compatibility of cholecalciferol, haloperidol, imipramine hydrochloride, levodopa/carbidopa, lorazepam, minocycline hydrochloride, tacrolimus monohydrate, terbinafine, tramadol hydrochloride and valsartan in SyrSpend SF PH4 oral suspensions.Pharmazie. 2016 Apr;71(4):185-91. Pharmazie. 2016. PMID: 27209697
-
Compatibility of Baclofen, Carvedilol, Hydrochlorothiazide, Mercaptopurine, Methadone Hydrochloride, Oseltamivir Phosphate, Phenobarbital, Propranolol Hydrochloride, Pyrazinamide, Sotalol Hydrochloride, Spironolactone, Tacrolimus Monohydrate, Ursodeoxycholic Acid, and Vancomycin Hydrochloride Oral Suspensions Compounded with SyrSpend SF pH4.Int J Pharm Compd. 2018 Nov-Dec;22(6):516-526. Int J Pharm Compd. 2018. PMID: 30384353
-
Physicochemical and Microbiological Stability of Amitriptyline Hydrochloride Oral Liquid Dosage Forms in PCCA Base, SuspendIt.Int J Pharm Compd. 2022 Jul-Aug;26(4):342-351. Int J Pharm Compd. 2022. PMID: 35820140 Review.
-
Physicochemical and Microbiological Stability of Extemporaneously Compounded Hydrocortisone Oral Suspensions in PCCA Base, SuspendIt.Int J Pharm Compd. 2021 Sep-Oct;25(5):431-439. Int J Pharm Compd. 2021. PMID: 34623970 Review.
Cited by
-
Accuracy of Dose Administered to Children Using Off-Labelled or Unlicensed Oral Dosage Forms.Pharmaceutics. 2021 Jul 2;13(7):1014. doi: 10.3390/pharmaceutics13071014. Pharmaceutics. 2021. PMID: 34371705 Free PMC article.
-
Characterization of Liquid Dosage Forms of Atenolol and Enalapril Maleate for Oral and Enteral Feeding Administration.Pharmaceuticals (Basel). 2024 Aug 9;17(8):1052. doi: 10.3390/ph17081052. Pharmaceuticals (Basel). 2024. PMID: 39204157 Free PMC article.
-
Safety and Biopharmaceutical Challenges of Excipients in Off-Label Pediatric Formulations.Int J Gen Med. 2020 Nov 9;13:1051-1066. doi: 10.2147/IJGM.S280330. eCollection 2020. Int J Gen Med. 2020. PMID: 33204140 Free PMC article. Review.
-
Oral delivery of peptide therapeutics in infants: Challenges and opportunities.Adv Drug Deliv Rev. 2021 Jun;173:112-124. doi: 10.1016/j.addr.2021.03.011. Epub 2021 Mar 25. Adv Drug Deliv Rev. 2021. PMID: 33774115 Free PMC article. Review.
-
Spotlight on Spironolactone Oral Suspension for the Treatment of Heart Failure: Focus on Patient Selection and Perspectives.Vasc Health Risk Manag. 2019 Dec 30;15:571-579. doi: 10.2147/VHRM.S210150. eCollection 2019. Vasc Health Risk Manag. 2019. PMID: 31920323 Free PMC article. Review.
References
-
- Nahata M.C. Lack of Pediatric Drug Formulations. Pediatrics. 1999;104:607–609. - PubMed
-
- Conroy S., Choonara I., Impicciatore P., Mohn A., Arnell H., Rane A., Knoeppel C., Seyberth H., Pandolfini C., Raffaelli M.P., et al. Survey of unlicensed and off label drug use in paediatric wards in European countries. European Network for Drug Investigation in Children. BMJ. 2000;320:79–82. doi: 10.1136/bmj.320.7227.79. - DOI - PMC - PubMed
-
- European Medicines Agency Reflection Paper: Formulation of Choice for the Paediatric Population. [(accessed on 14 March 2019)];2006 Available online: https://www.ema.europa.eu/documents/scientific-guideline/reflection-pape....
-
- Richey R.H., Shah U.U., Peak M., Craig J.V., Ford J.L., Barker C.E., Nunn A.J., Turner M.A. Manipulation of drugs to achieve the required dose is intrinsic to paediatric practice but is not supported by guidelines or evidence. BMC Pediatr. 2013;13:81. doi: 10.1186/1471-2431-13-81. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

