Trastuzumab-Modified Gold Nanoparticles Labeled with 211At as a Prospective Tool for Local Treatment of HER2-Positive Breast Cancer
- PMID: 31003512
- PMCID: PMC6523862
- DOI: 10.3390/nano9040632
Trastuzumab-Modified Gold Nanoparticles Labeled with 211At as a Prospective Tool for Local Treatment of HER2-Positive Breast Cancer
Abstract
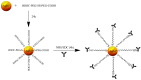
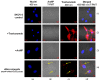
Highly localized radiotherapy with radionuclides is a commonly used treatment modality for patients with unresectable solid tumors. Herein, we propose a novel α-nanobrachytherapy approach for selective therapy of human epidermal growth factor receptor 2 (HER2)-positive breast cancer. This uses local intratumoral injection of 5-nm-diameter gold nanoparticles (AuNPs) labeled with an α-emitter (211At), modified with polyethylene glycol (PEG) chains and attached to HER2-specific monoclonal antibody (trastuzumab). The size, shape, morphology, and zeta potential of the 5 nm synthesized AuNPs were characterized by TEM (Transmission Electron Microscopy) and DLS (Dynamic Light Scattering) techniques. The gold nanoparticle surface was modified by PEG and subsequently used for antibody immobilization. Utilizing the high affinity of gold for heavy halogens, the bioconjugate was labelled with 211At obtained by α irradiation of the bismuth target. The labeling yield of 211At was greater than 99%. 211At bioconjugates were stable in human serum. Additionally, in vitro biological studies indicated that 211At-AuNP-PEG-trastuzumab exhibited higher affinity and cytotoxicity towards the HER2-overexpressing human ovarian SKOV-3 cell line than unmodified nanoparticles. Confocal and dark field microscopy studies revealed that 211At-AuNP-PEG-trastuzumab was effectively internalized and deposited near the nucleus. These findings show promising potential for the 211At-AuNP-PEG-trastuzumab radiobioconjugate as a perspective therapeutic agent in the treatment of unresectable solid cancers expressing HER2 receptors.
Keywords: HER2 receptor; SKOV-3 ovarian cell line; monoclonal antibody; α-emitter 211At; α-nanobrachytherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

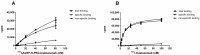

References
-
- Gerbaulet A., Pötter R., Mazeron J.J., HMeertens H., Van Limbergen E. The GEC ESTRO Handbook of Brachytherapy. 2nd ed. European Society for Therapeutic Radiology and Oncology; Leuven, Belgium: 2002.
-
- Li J., Zhang L., Xie Q., Wang W., Hua Y., Sun Z. Comparison of clinical efficacy and complications of 125I seed brachytherapy and stereotactic body radiation therapy for recurrent pulmonary metastases from colorectal carcinoma. J. Contemp. Brachytherapy. 2018;10:360–367. doi: 10.5114/jcb.2018.77956. - DOI - PMC - PubMed
-
- Parashar B., Wernicke A.G., Pavese A., Singh P., Trichter S., Sabbas A., Kutler D.I., Kuhe W., Port J.L., Lee P.C., et al. Cesium-131 permanent seed brachytherapy: Dosimetric evaluation and radiation exposure to surgeons, radiation oncologists, and staff. Brachytherapy. 2011;10:508–513. doi: 10.1016/j.brachy.2011.04.002. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

