TRPC3 Regulates the Proliferation and Apoptosis Resistance of Triple Negative Breast Cancer Cells through the TRPC3/RASA4/MAPK Pathway
- PMID: 31003514
- PMCID: PMC6520729
- DOI: 10.3390/cancers11040558
TRPC3 Regulates the Proliferation and Apoptosis Resistance of Triple Negative Breast Cancer Cells through the TRPC3/RASA4/MAPK Pathway
Abstract
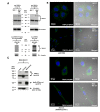
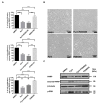
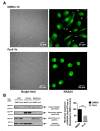
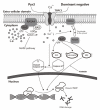
Currently, there is no effective molecular-based therapy for triple-negative breast cancer (TNBC). Canonical transient receptor potential isoform 3 (TRPC3) was previously shown to be upregulated in breast cancer biopsy tissues when compared to normal breast tissues. However, the biological role of TRPC3 in breast cancer still remains to be elucidated. In this study, subcellular fractionation followed by Western blot and immunocytochemistry showed that TRPC3 was over-expressed on the plasma membrane of TNBC line MDA-MB-231 when compared to an estrogen receptor-positive cell line MCF-7. TRPC3 blocker Pyr3 and dominant negative of TRPC3 attenuated proliferation, induced apoptosis and sensitized cell death to chemotherapeutic agents in MDA-MB-231 as measured by proliferation assays. Interestingly, Ras GTPase-activating protein 4 (RASA4), a Ca2+-promoted Ras-MAPK pathway suppressor, was found to be located on the plasma membrane of MDA-MB-231. Blocking TRPC3 decreased the amount of RASA4 located on the plasma membrane, with concomitant activation of MAPK pathways. Our results suggest that, in TNBC MDA-MB-231 cells, Ca2+ influx through TRPC3 channel sustains the presence of RASA4 on the plasma membrane where it inhibits the Ras-MAPK pathway, leading to proliferation and apoptosis resistance. Our study reveals the novel TRPC3-RASA4-MAPK signaling cascade in TNBC cells and suggests that TRPC3 may be exploited as a potential therapeutic target for TNBC.
Keywords: MAPK pathway; RASA4; TRPC3; apoptosis resistance; calcium influx; triple-negative breast cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
TRPC3-mediated NFATc1 calcium signaling promotes triple negative breast cancer migration through regulating glypican-6 and focal adhesion.Pflugers Arch. 2025 Feb;477(2):253-272. doi: 10.1007/s00424-024-03030-y. Epub 2024 Oct 22. Pflugers Arch. 2025. PMID: 39436410 Free PMC article.
-
Induction of AMPK activation by N,N'-diarylurea FND-4b decreases growth and increases apoptosis in triple negative and estrogen-receptor positive breast cancers.PLoS One. 2019 Mar 15;14(3):e0209392. doi: 10.1371/journal.pone.0209392. eCollection 2019. PLoS One. 2019. PMID: 30875375 Free PMC article.
-
Tryptophan hydroxylase 1 and 5-HT7 receptor preferentially expressed in triple-negative breast cancer promote cancer progression through autocrine serotonin signaling.Mol Cancer. 2016 Nov 21;15(1):75. doi: 10.1186/s12943-016-0559-6. Mol Cancer. 2016. PMID: 27871326 Free PMC article.
-
RASA4 inhibits the HIFα signaling pathway to suppress proliferation of cervical cancer cells.Bioengineered. 2021 Dec;12(2):10723-10733. doi: 10.1080/21655979.2021.2002499. Bioengineered. 2021. PMID: 34752201 Free PMC article.
-
Estrogen receptor-α36 is involved in icaritin induced growth inhibition of triple-negative breast cancer cells.J Steroid Biochem Mol Biol. 2017 Jul;171:318-327. doi: 10.1016/j.jsbmb.2017.05.009. Epub 2017 May 18. J Steroid Biochem Mol Biol. 2017. PMID: 28529129
Cited by
-
A novel strategy for treating cancer: understanding the role of Ca2+ signaling from nociceptive TRP channels in regulating cancer progression.Explor Target Antitumor Ther. 2021;2(5):401-415. doi: 10.37349/etat.2021.00053. Epub 2021 Oct 31. Explor Target Antitumor Ther. 2021. PMID: 36045706 Free PMC article. Review.
-
Integrative analysis of exosomal ncRNAs and their regulatory networks in liver cancer progression.Pract Lab Med. 2025 Mar 21;45:e00464. doi: 10.1016/j.plabm.2025.e00464. eCollection 2025 Jul. Pract Lab Med. 2025. PMID: 40226122 Free PMC article.
-
DNA methylation in monozygotic twins discordant for acute lymphoblastic leukemia: a case report and systematic review.Clin Epigenetics. 2025 Jun 6;17(1):94. doi: 10.1186/s13148-025-01906-z. Clin Epigenetics. 2025. PMID: 40481591 Free PMC article. Review.
-
Roles of calcium signaling in cancer metastasis to bone.Explor Target Antitumor Ther. 2022;3(4):445-462. doi: 10.37349/etat.2022.00094. Epub 2022 Aug 31. Explor Target Antitumor Ther. 2022. PMID: 36071984 Free PMC article. Review.
-
Discovery of a Highly Selective and Potent TRPC3 Inhibitor with High Metabolic Stability and Low Toxicity.ACS Med Chem Lett. 2021 Mar 5;12(4):572-578. doi: 10.1021/acsmedchemlett.0c00571. eCollection 2021 Apr 8. ACS Med Chem Lett. 2021. PMID: 33859797 Free PMC article.
References
-
- Galluzzi L., Vitale I., Aaronson S.A., Abrams J.M., Adam D., Agostinis P., Alnemri E.S., Altucci L., Amelio I., Andrews D.W., et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

