E arly Lifestyle Interventions in People with Impaired Glucose Tolerance in Northern Colombia: The DEMOJUAN Project
- PMID: 31003515
- PMCID: PMC6518277
- DOI: 10.3390/ijerph16081403
E arly Lifestyle Interventions in People with Impaired Glucose Tolerance in Northern Colombia: The DEMOJUAN Project
Abstract
Background: The objective of the demonstration project for type 2 diabetes prevention in the Barranquilla and Juan Mina (DEMOJUAN) study was to investigate the extent to which it is possible to reach normal glucose metabolism with early lifestyle interventions in people at high risk of type 2 diabetes (prediabetes), compared with those who receive standard usual care.
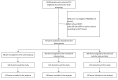
Methods: DEMOJUAN was a randomized controlled trial conducted in Juan Mina and Barranquilla, Northern Colombia. Eligible participants were randomized into one of three groups (control group, initial nutritional intervention, and initial physical activity intervention). The duration of the intervention was 24 months. The main study outcome in the present analysis was reversion to normoglycemia. Relative risks and their corresponding 95% confidence intervals were calculated for reversal to normoglycemia and T2D incidence.
Results: There was no statistically significant association between the intervention groups and reversion to normoglycemia. The relative risk of reversion to normoglycemia was 0.88 (95% CI 0.70-1.12) for the initial nutritional intervention group participants and 0.95 (95% CI 0.75-1.20) for the initial physical activity intervention group participants.
Conclusions: Our study did not find any statistically significant differences in reversion to normoglycemia or the development of type 2 diabetes between the intervention groups and the control group in this population.
Keywords: South America; field trial; glucose metabolism disorders; population; primary prevention.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Diabetes Atlas. 7th ed. International Diabetes Federation (IDF); Brussels, Belgium: 2017.
-
- Pan X.R., Li G.W., Hu Y.H., Wang J.X., Yang W.Y., An Z.X., Hu Z.X., Lin J., Xiao J.Z., Cao H.B., et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. - DOI - PubMed
-
- Ramachandran A., Snehalatha C., Mary S., Mukesh B., Bhaskar A.D., Vijay V., Indian Diabetes Prevention Programme (IDPP) The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1) Diabetologia. 2006;4:289–297. doi: 10.1007/s00125-005-0097-z. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical