Colitis-Induced Th17 Cells Increase the Risk for Severe Subsequent Clostridium difficile Infection
- PMID: 31003940
- PMCID: PMC6509008
- DOI: 10.1016/j.chom.2019.03.003
Colitis-Induced Th17 Cells Increase the Risk for Severe Subsequent Clostridium difficile Infection
Abstract
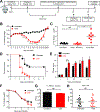
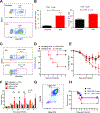
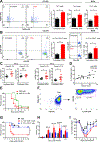
Clostridium difficile infection (CDI) is the number one hospital-acquired infection in the United States. CDI is more common and severe in inflammatory bowel disease patients. Here, we studied the mechanism by which prior colitis exacerbates CDI. Mice were given dextran sulfate sodium (DSS) colitis, recovered for 2 weeks, and then were infected with C. difficile. Mortality and CDI severity were increased in DSS-treated mice compared to controls. Severe CDI is dependent on CD4+ T cells, which persist after colitis-associated inflammation subsides. Adoptive transfer of Th17 cells to naive mice is sufficient to increase CDI-associated mortality through elevated IL-17 production. Finally, in humans, the Th17 cytokines IL-6 and IL-23 associate with severe CDI, and patients with high serum IL-6 are 7.6 times more likely to die post infection. These findings establish a central role for Th17 cells in CDI pathogenesis following colitis and identify them as a potential target for preventing severe disease.
Keywords: CD4 T cells; Clostridium difficile; DSS; IL-17A; IL-23; IL-6; Th17 cells; adaptive immunity; colitis; inflammatory bowel disease.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
W.A.P. is a consultant for TechLab, a company that makes diagnostics for CDI. Some of the data in this manuscript was used to file U.S. Provisional Patent Application Serial No. 62/630,370.
Figures

Comment in
-
Intestinal Damage Fades, but Insults Linger: Setting the Immunological Tone for Infection.Cell Host Microbe. 2019 May 8;25(5):636-637. doi: 10.1016/j.chom.2019.04.008. Cell Host Microbe. 2019. PMID: 31071292
References
-
- Ahern PP, Izcue A, Maloy KJ, and Powrie F (2008). The interleukin-23 axis in intestinal inflammation. Immunological Reviews 226, 147–159. - PubMed
-
- Ananthakrishnan AN, McGinley EL, and Binion DG (2008). Excess hospitalisation burden associated with Clostridium difficile in patients with inflammatory bowel disease. Gut 57, 205–210. - PubMed
-
- Arai Y, Takanashi H, Kitagawa H, and Okayasu I (1998). Involvement of interleukin-1 in the development of ulcerative colitis induced by dextran sulfate sodium in mice. Cytokine 10, 890–896. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

