Identification of functional lipid metabolism biomarkers of brown adipose tissue aging
- PMID: 31003944
- PMCID: PMC6531832
- DOI: 10.1016/j.molmet.2019.03.011
Identification of functional lipid metabolism biomarkers of brown adipose tissue aging
Abstract
Objective: Aging is accompanied by loss of brown adipocytes and a decline in their thermogenic potential, which may exacerbate the development of adiposity and other metabolic disorders. Presently, only limited evidence exists describing the molecular alterations leading to impaired brown adipogenesis with aging and the contribution of these processes to changes of systemic energy metabolism.
Methods: Samples of young and aged murine brown and white adipose tissue were used to compare age-related changes of brown adipogenic gene expression and thermogenesis-related lipid mobilization. To identify potential markers of brown adipose tissue aging, non-targeted proteomic and metabolomic as well as targeted lipid analyses were conducted on young and aged tissue samples. Subsequently, the effects of several candidate lipid classes on brown adipocyte function were examined.
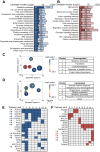
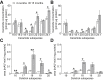
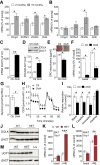
Results: Corroborating previous reports of reduced expression of uncoupling protein-1, we observe impaired signaling required for lipid mobilization in aged brown fat after adrenergic stimulation. Omics analyses additionally confirm the age-related impairment of lipid homeostasis and reveal the accumulation of specific lipid classes, including certain sphingolipids, ceramides, and dolichols in aged brown fat. While ceramides as well as enzymes of dolichol metabolism inhibit brown adipogenesis, inhibition of sphingosine 1-phosphate receptor 2 induces brown adipocyte differentiation.
Conclusions: Our functional analyses show that changes in specific lipid species, as observed during aging, may contribute to reduced thermogenic potential. They thus uncover potential biomarkers of aging as well as molecular mechanisms that could contribute to the degradation of brown adipocytes, thereby providing potential treatment strategies of age-related metabolic conditions.
Keywords: Aging; Brown adipose tissue; Ceramides; Dolichol lipids; Sphingolipids.
Copyright © 2019 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures




Similar articles
-
Identification of biomarkers of brown adipose tissue aging highlights the role of dysfunctional energy and nucleotide metabolism pathways.Sci Rep. 2021 Oct 7;11(1):19928. doi: 10.1038/s41598-021-99362-1. Sci Rep. 2021. PMID: 34620947 Free PMC article.
-
Ceramides are necessary and sufficient for diet-induced impairment of thermogenic adipocytes.Mol Metab. 2021 Mar;45:101145. doi: 10.1016/j.molmet.2020.101145. Epub 2020 Dec 19. Mol Metab. 2021. PMID: 33352310 Free PMC article.
-
miR-199a-3p regulates brown adipocyte differentiation through mTOR signaling pathway.Mol Cell Endocrinol. 2018 Nov 15;476:155-164. doi: 10.1016/j.mce.2018.05.005. Epub 2018 Jun 28. Mol Cell Endocrinol. 2018. PMID: 29753771
-
Mechanisms of aging-related impairment of brown adipocyte development and function.Gerontology. 2015;61(3):211-7. doi: 10.1159/000366557. Epub 2014 Dec 20. Gerontology. 2015. PMID: 25531079 Review.
-
Molecular pathways regulating the formation of brown-like adipocytes in white adipose tissue.Diabetes Metab Res Rev. 2015 Jul;31(5):433-52. doi: 10.1002/dmrr.2600. Epub 2014 Oct 18. Diabetes Metab Res Rev. 2015. PMID: 25139773 Review.
Cited by
-
Aging selectively dampens oscillation of lipid abundance in white and brown adipose tissue.Sci Rep. 2021 Mar 15;11(1):5932. doi: 10.1038/s41598-021-85455-4. Sci Rep. 2021. PMID: 33723320 Free PMC article.
-
Lipid remodeling of adipose tissue in metabolic health and disease.Exp Mol Med. 2023 Sep;55(9):1955-1973. doi: 10.1038/s12276-023-01071-4. Epub 2023 Sep 1. Exp Mol Med. 2023. PMID: 37653032 Free PMC article. Review.
-
Contribution of specific ceramides to obesity-associated metabolic diseases.Cell Mol Life Sci. 2022 Jul 5;79(8):395. doi: 10.1007/s00018-022-04401-3. Cell Mol Life Sci. 2022. PMID: 35789435 Free PMC article. Review.
-
Effects of Lipids and Lipoproteins on Mesenchymal Stem Cells Used in Cardiac Tissue Regeneration.Int J Mol Sci. 2020 Jul 5;21(13):4770. doi: 10.3390/ijms21134770. Int J Mol Sci. 2020. PMID: 32635662 Free PMC article. Review.
-
Identification of biomarkers of brown adipose tissue aging highlights the role of dysfunctional energy and nucleotide metabolism pathways.Sci Rep. 2021 Oct 7;11(1):19928. doi: 10.1038/s41598-021-99362-1. Sci Rep. 2021. PMID: 34620947 Free PMC article.
References
-
- Cannon B., Nedergaard J. Respiratory and thermogenic capacities of cells and mitochondria from brown and white adipose tissue. Methods in Molecular Biology. 2001;155:295–303. - PubMed
-
- Richard D., Picard F. Brown fat biology and thermogenesis. Frontiers in Bioscience (Landmark Edition) 2011;16:1233–1260. - PubMed
-
- Nedergaard J., Bengtsson T., Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. American Journal of Physiology Endocrinology and Metabolism. 2007;293:E444–E452. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

