Use of immunosuppressive therapy for management of myelodysplastic syndromes: a systematic review and meta-analysis
- PMID: 31004015
- PMCID: PMC6939518
- DOI: 10.3324/haematol.2019.219345
Use of immunosuppressive therapy for management of myelodysplastic syndromes: a systematic review and meta-analysis
Abstract
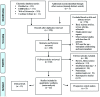
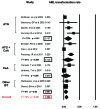
Immunosuppressive therapy (IST) is one therapy option for treatment of patients with lower-risk myelodysplastic syndromes (MDS). However, the use of several different immunosuppressive regimens, the lack of high-quality studies, and the absence of validated predictive biomarkers pose important challenges. We conducted a systematic review and meta-analysis according to the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines and searched MEDLINE via PubMed, Ovid EMBASE, COCHRANE registry of clinical trials (CENTRAL), and the Web of Science without language restriction from inception through September 2018, as well as relevant conference proceedings and abstracts, for prospective cohort studies or clinical trials investigating IST in MDS. Fixed and Random-effects models were used to pool response rates. We identified nine prospective cohort studies and 13 clinical trials with a total of 570 patients. Overall response rate was 42.5% [95% confidence interval (CI): 36.1-49.2%] including a complete remission rate of 12.5% (95%CI: 9.3-16.6%) and red blood cell transfusion independence rate of 33.4% (95% CI: 25.1-42.9%). The most commonly used forms of IST were anti-thymocyte globulin alone or in combination with cyclosporin A with a trend towards higher response rates with combination therapy. Progression rate to acute myeloid leukemia was 8.6% per patient year (95%CI: 3.3-13.9%). Overall survival and adverse events were only inconsistently reported. We were unable to validate any biomarkers predictive of a therapeutic response to IST. IST for treatment of lower-risk MDS patients can be successful to alleviate transfusion burden and associated sequelae.
Copyright© 2020 Ferrata Storti Foundation.
Figures


Similar articles
-
Immunosuppressive therapy for myelodysplastic syndromes: refining the indications.Curr Hematol Malig Rep. 2008 Jan;3(1):23-8. doi: 10.1007/s11899-008-0005-y. Curr Hematol Malig Rep. 2008. PMID: 20425443
-
Immunosuppressive Therapy: Exploring an Underutilized Treatment Option for Myelodysplastic Syndrome.Clin Lymphoma Myeloma Leuk. 2016 Aug;16 Suppl:S44-8. doi: 10.1016/j.clml.2016.02.017. Clin Lymphoma Myeloma Leuk. 2016. PMID: 27521323
-
The use of immunosuppressive therapy in MDS: clinical outcomes and their predictors in a large international patient cohort.Blood Adv. 2018 Jul 24;2(14):1765-1772. doi: 10.1182/bloodadvances.2018019414. Blood Adv. 2018. PMID: 30037803 Free PMC article. Clinical Trial.
-
Systematic review and meta-analysis of the effect of iron chelation therapy on overall survival and disease progression in patients with lower-risk myelodysplastic syndromes.Ann Hematol. 2019 Feb;98(2):339-350. doi: 10.1007/s00277-018-3539-7. Epub 2018 Nov 9. Ann Hematol. 2019. PMID: 30413901
-
The efficacy and adverse events of venetoclax in combination with hypomethylating agents treatment for patients with acute myeloid leukemia and myelodysplastic syndrome: a systematic review and meta-analysis.Hematology. 2020 Dec;25(1):414-423. doi: 10.1080/16078454.2020.1843752. Hematology. 2020. PMID: 33191860
Cited by
-
Interferon alpha therapy in essential thrombocythemia and polycythemia vera-a systematic review and meta-analysis.Leukemia. 2021 Jun;35(6):1643-1660. doi: 10.1038/s41375-020-01020-4. Epub 2020 Sep 1. Leukemia. 2021. PMID: 32868875 Free PMC article.
-
Use of steroids in the management of low-risk myelodysplastic syndromes with autoimmune features.Blood Transfus. 2023 Sep;21(5):452-460. doi: 10.2450/2022.0184-22. Epub 2022 Dec 22. Blood Transfus. 2023. PMID: 36580026 Free PMC article.
-
Emerging treatment options for patients with high-risk myelodysplastic syndrome.Ther Adv Hematol. 2020 Nov 11;11:2040620720955006. doi: 10.1177/2040620720955006. eCollection 2020. Ther Adv Hematol. 2020. PMID: 33240476 Free PMC article. Review.
-
Current Therapy of the Patients with MDS: Walking towards Personalized Therapy.J Clin Med. 2021 May 13;10(10):2107. doi: 10.3390/jcm10102107. J Clin Med. 2021. PMID: 34068316 Free PMC article. Review.
-
Past, present and future in low-risk myelodysplastic syndrome.Front Med (Lausanne). 2022 Jul 15;9:967900. doi: 10.3389/fmed.2022.967900. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35911422 Free PMC article. Review.
References
-
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. - PubMed
-
- Swerdlow SH, Harris NL, Jaffe ES, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. WHO Classification of Tumours, Revised 4th Edition. 2017; Volume 2.
-
- Greenberg PL, Stone RM, Al-Kali A, et al. Myelodysplastic Syndromes, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15(1):60–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

