Onset of efficacy and duration of response of galcanezumab for the prevention of episodic migraine: a post-hoc analysis
- PMID: 31004075
- PMCID: PMC6691875
- DOI: 10.1136/jnnp-2018-320242
Onset of efficacy and duration of response of galcanezumab for the prevention of episodic migraine: a post-hoc analysis
Abstract
Background and objective: As new migraine prevention treatments are developed, the onset of a preventive effect, how long it is maintained and whether patients initially non-responsive develop clinically meaningful responses with continued treatment can be assessed.
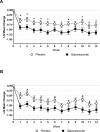
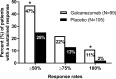
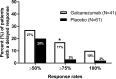
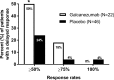
Methods: Analyses were conducted post-hoc of a double-blind, placebo-controlled, phase II-a study in patients with episodic migraine receiving galcanezumab 150 mg or placebo biweekly for 12 weeks (Lancet Neurol 13:885, 2014). The number of migraine headache days per week, and onset of efficacy measured as the first week galacanezumab separated from placebo were determined. Patients with ≥50%, ≥75% and 100% reduction in migraine headache days from baseline at months 1, 2 and 3 were calculated and defined as sustained responses. Non-responders (<50% response) at month 1 or 2 who then showed ≥50%, ≥75% and 100% response at later time-points were calculated.
Results: Patients were randomised to galcanezumab (n=107) or placebo (n=110). A significant (p=0.018) change of -0.89±0.11 (galcanezumab) vs -0.53±0.11 (placebo) migraine headache days indicated onset at week 1. Forty-seven per cent of galcanezumab and 25% of placebo patients responding at month 1 maintained response through months 2 and 3. Of non-responders at month 1, 27% on galcanezumab and 20% on placebo responded on months 2 and 3, and 50% of galcanezumab non-responders in months 1 and 2 responded on month 3, vs 24% on placebo.
Conclusions: The onset of efficacy of galcanezumab is within 1 week in a majority of patients, and patients receiving galcanezumab are twice more likely to maintain responses than placebo patients. Early non-responders may respond by month 2 or month 3.
Trial registration number: NCT01625988.
Keywords: CGRP; LY2951742; calcitonin gene-related peptide; clinical trial; galcanezumab; migraine.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: The studies included in the analyses were sponsored and/or supported by Eli Lilly and Company. VS, MBF, JMM, TMO and SKA are full-time employees of Eli Lilly and Company and/or one of its subsidiaries, and are stockholders. QZ was an employee of Eli Lilly and Company at the time the manuscript was written and is presently an employee of Sanofi and is an Eli Lilly and Company stockholder. PJG reports grants and personal fees from Amgen and Eli Lilly and Company; personal fees from Alder BioPharmaceuticals, Allergan, Autonomic Technologies, Dr Reddy’s Laboratories, Biohaven Pharmaceuticals, Electrocore, eNeura, Novartis,Impel Neuropharma, Mundipharma, Teva Pharmaceuticals and Trigemina; and personal fees from MedicoLegal work, UptoDate, Oxford University Press, Massachusetts Medical Society and Wolters Kluwer; and a patent magnetic stimulation for headache assigned to eNeura without fee. Within the last 12 months, DWD reports personal fees from Amgen, lder, Allergan, Autonomic Technologies, Biohaven, Eli Lilly, eNeura, Foresight Capital, Neurolief, Zosano, WL Gore, Vedanta Associates, Promius Pharma, Nocira, Novartis, Electrocore, Teva, Ipsen, Impel, Satsuma, Charleston Laboratories and Theranica; compensation for activities related to data safety monitoring committee from Axsome; compensation related to CME content development: HealthLogiX, Medicom Worldwide, MedLogix Communications, MedNet, Miller Medical Communications, PeerView Operation Services America, WebMD/Medscape, American Academy of Neurology, American Headache Society, PeerView Institute for Medical Education, Chameleon Communications, Academy for Continued Healthcare Learning, Universal Meeting Management, Haymarket Medical Education, Global Scientific Communications, UpToDate and Meeting LogiX; royalties from editorial or book publishing: Oxford University Press, Cambridge University Press, Wiley-Blackwell, Sage and Wolters Kluwer Health; consulting use agreement through employer: Neuro Assessment Systems and Myndshft; holds equity in Aural Analytics, Healint, Theranica, Second Opinion/Mobile Health and Epien; and board of directors position: King-Devick Technologies and Ontologics.
Figures

References
-
- Disease GBD, Injury I, Global PC, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the global burden of Disease Study 2016. Lancet 2017;390:1211–59. 10.1016/S0140-6736(17)32154-2 - DOI - PMC - PubMed
-
- Goadsby PJ, Sprenger T. Current practice and future directions in the management of migraine: acute and preventive. Lancet Neurology 2010;9:285–98. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
