Exposure to aristolochic acid I compromises the maturational competency of porcine oocytes via oxidative stress-induced DNA damage
- PMID: 31004078
- PMCID: PMC6520013
- DOI: 10.18632/aging.101911
Exposure to aristolochic acid I compromises the maturational competency of porcine oocytes via oxidative stress-induced DNA damage
Abstract
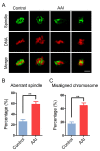
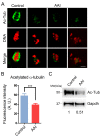
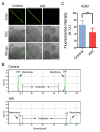
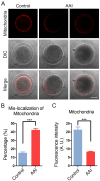
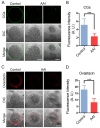
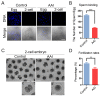
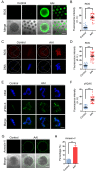
Aristolochic acid (AA) is a class of carcinogenic and nephrotoxic nitrophenanthrene carboxylic acids naturally found in Aristolochia plants. These plants have been widely used as herbal medicines and also enter the human food chain as the persistent soil pollutants. It has been known that AA exposure is implicated in multiple cancer types, kidney failure and ovarian dysfunction. However, whether AA exposure would influence the oocyte quality has not yet determined. Here, we document that AAI has the negative effects on the competency of oocyte maturation and fertilization. We show that AAI exposure leads to the oocyte meiotic failure via impairing the meiotic apparatus, displaying a prominently defective spindle assembly, actin dynamics and mitochondrial integrity. AAI exposure also causes the abnormal distribution of cortical granules and ovastacin, which is consistent with the observation that fewer sperm bound to the zona pellucida surrounding the unfertilized AAI-exposed eggs, contributing to the fertilization failure. In addition, AAI exposure induces the increased levels of ROS, DNA damage and early apoptosis in porcine oocytes. Collectively, we demonstrate that AAI exposure perturbs the oocyte meiotic progression and fertilization capacity via disruption of both nuclear maturation and cytoplasmic maturation of oocyte, which might be caused by the excessive oxidative stress-induced DNA damage and apoptosis.
Keywords: Aristolochic acid; DNA damage; fertilization capacity; oocyte maturation; oxidative stress.
Conflict of interest statement
Figures

Similar articles
-
The protective role of melatonin in porcine oocyte meiotic failure caused by the exposure to benzo(a)pyrene.Hum Reprod. 2018 Jan 1;33(1):116-127. doi: 10.1093/humrep/dex331. Hum Reprod. 2018. PMID: 29112712
-
BaP exposure causes oocyte meiotic arrest and fertilization failure to weaken female fertility.FASEB J. 2018 Jan;32(1):342-352. doi: 10.1096/fj.201700514R. Epub 2017 Sep 13. FASEB J. 2018. PMID: 28904021
-
Exposure to perfluorooctane sulfonate in vitro perturbs the quality of porcine oocytes via induction of apoptosis.Environ Pollut. 2021 Sep 1;284:117508. doi: 10.1016/j.envpol.2021.117508. Epub 2021 Jun 2. Environ Pollut. 2021. PMID: 34261219
-
How Do Environmental Toxicants Affect Oocyte Maturation Via Oxidative Stress?Adv Anat Embryol Cell Biol. 2024;238:69-95. doi: 10.1007/978-3-031-55163-5_4. Adv Anat Embryol Cell Biol. 2024. PMID: 39030355 Review.
-
Oxidative Stress and Oocyte Cryopreservation: Recent Advances in Mitigation Strategies Involving Antioxidants.Cells. 2022 Nov 11;11(22):3573. doi: 10.3390/cells11223573. Cells. 2022. PMID: 36429002 Free PMC article. Review.
Cited by
-
The Role of Ca2 + in Maturation and Reprogramming of Bovine Oocytes: A System Study of Low-Calcium Model.Front Cell Dev Biol. 2021 Oct 26;9:746237. doi: 10.3389/fcell.2021.746237. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34765601 Free PMC article.
-
Hexestrol Deteriorates Oocyte Quality via Perturbation of Mitochondrial Dynamics and Function.Front Cell Dev Biol. 2021 Jul 6;9:708980. doi: 10.3389/fcell.2021.708980. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34295902 Free PMC article.
-
Aristolochic acid I exposure decreases oocyte quality.Front Cell Dev Biol. 2022 Aug 11;10:838992. doi: 10.3389/fcell.2022.838992. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36036003 Free PMC article.
-
Salidroside Supplementation Affects In Vitro Maturation and Preimplantation Embryonic Development by Promoting Meiotic Resumption.Genes (Basel). 2023 Aug 30;14(9):1729. doi: 10.3390/genes14091729. Genes (Basel). 2023. PMID: 37761869 Free PMC article.
-
Beneficial Effects of Catalpol Supplementation during In Vitro Maturation of Porcine Cumulus-Oocyte Complexes.Antioxidants (Basel). 2023 Jun 5;12(6):1222. doi: 10.3390/antiox12061222. Antioxidants (Basel). 2023. PMID: 37371952 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

