Fingerprinting of Proteins that Mediate Quagga Mussel Adhesion using a De Novo Assembled Foot Transcriptome
- PMID: 31004089
- PMCID: PMC6474901
- DOI: 10.1038/s41598-019-41976-7
Fingerprinting of Proteins that Mediate Quagga Mussel Adhesion using a De Novo Assembled Foot Transcriptome
Abstract
The European freshwater mollusk Dreissena bugensis (quagga mussel), an invasive species to North America, adheres to surfaces underwater via the byssus: a non-living protein 'anchor'. In spite of its importance as a biofouling species, the sequence of the majority of byssal proteins responsible for adhesion are not known, and little genomic data is available. To determine protein sequence information, we utilized next-generation RNA sequencing and de novo assembly to construct a cDNA library of the quagga mussel foot transcriptome, which contains over 200,000 transcripts. Quagga mussel byssal proteins were extracted from freshly induced secretions and analyzed using LC-MS/MS; peptide spectra were matched to the transcriptome to fingerprint the entire protein primary sequences. We present the full sequences of fourteen novel quagga mussel byssal proteins, named Dreissena bugensis foot proteins 4 to 17 (Dbfp4-Dbfp17), and new sequence data for two previously observed byssal proteins Dbfp1 and Dbfp2. Theoretical masses of the newly discovered proteins range from 4.3 kDa to 21.6 kDa. These protein sequences are unique but contain features similar to glue proteins from other species, including a high degree of polymorphism, proteins with repeated peptide motifs, disordered protein structure, and block structures.
Conflict of interest statement
The authors declare no competing interests.
Figures
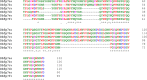

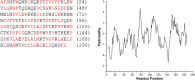
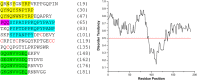

Similar articles
-
Spatial distribution of proteins in the quagga mussel adhesive apparatus.Biofouling. 2016;32(2):205-13. doi: 10.1080/08927014.2015.1135426. Biofouling. 2016. PMID: 26825294
-
Novel proteins identified in the insoluble byssal matrix of the freshwater zebra mussel.Mar Biotechnol (NY). 2014 Apr;16(2):144-55. doi: 10.1007/s10126-013-9537-9. Epub 2013 Sep 24. Mar Biotechnol (NY). 2014. PMID: 24057171
-
Byssal proteins of the freshwater zebra mussel, Dreissena polymorpha.Biofouling. 2013;29(1):77-85. doi: 10.1080/08927014.2012.746672. Biofouling. 2013. PMID: 23211030
-
The quagga mussel, Dreissena rostriformis: a novel model for EcoEvoDevo, environmental research, and the applied sciences.Front Cell Dev Biol. 2025 Jan 9;12:1531560. doi: 10.3389/fcell.2024.1531560. eCollection 2024. Front Cell Dev Biol. 2025. PMID: 39850802 Free PMC article. Review.
-
Review and Development of Best Practices for Toxicity Tests with Dreissenid Mussels.Environ Toxicol Chem. 2023 Aug;42(8):1649-1666. doi: 10.1002/etc.5648. Epub 2023 Jun 23. Environ Toxicol Chem. 2023. PMID: 37191358 Review.
Cited by
-
The venom and telopodal defence systems of the centipede Lithobius forficatus are functionally convergent serial homologues.BMC Biol. 2024 Jun 13;22(1):135. doi: 10.1186/s12915-024-01925-x. BMC Biol. 2024. PMID: 38867210 Free PMC article.
-
Invasive mussels fashion silk-like byssus via mechanical processing of massive horizontally acquired coiled coils.Proc Natl Acad Sci U S A. 2023 Nov 28;120(48):e2311901120. doi: 10.1073/pnas.2311901120. Epub 2023 Nov 20. Proc Natl Acad Sci U S A. 2023. PMID: 37983489 Free PMC article.
-
Structure, function and parallel evolution of the bivalve byssus, with insights from proteomes and the zebra mussel genome.Philos Trans R Soc Lond B Biol Sci. 2021 May 24;376(1825):20200155. doi: 10.1098/rstb.2020.0155. Epub 2021 Apr 5. Philos Trans R Soc Lond B Biol Sci. 2021. PMID: 33813897 Free PMC article.
-
Glycoproteins Involved in Sea Urchin Temporary Adhesion.Mar Drugs. 2023 Feb 24;21(3):145. doi: 10.3390/md21030145. Mar Drugs. 2023. PMID: 36976195 Free PMC article.
-
"Omics" Techniques Used in Marine Biofouling Studies.Int J Mol Sci. 2023 Jun 23;24(13):10518. doi: 10.3390/ijms241310518. Int J Mol Sci. 2023. PMID: 37445696 Free PMC article. Review.
References
-
- Karatayev AY, Claudi R, Lucy FE. History of Dreissena Research and the ICAIS Gateway to Aquatic Invasions Science. International Conference on Aquatic Invasive Species. 2010;7(1):1–5.
-
- Hebert PDN, Muncaster BW, Mackie GL. Ecological and Genetic Studies on Dreissena polymorpha (Pallas): a New Mollusc in the Great Lakes. Canadian Journal of Fisheries and Aquatic Sciences. 1989;46(9):1587–1591. doi: 10.1139/f89-202. - DOI
-
- Mills EL, et al. A Review of the Biology and Ecology of the Quagga Mussel (Dreissena bugensis), a Second Species of Freshwater Dreissenid Introduced to North America. Integrative and Comparative Biology. 1996;36(3):271–286.
-
- Ackerman JD, Cottrell CM, Ethier CR, Allen DG. Attachment Strength of Zebra Mussels on Natural, Polymeric, and Metallic Materials. Journal of Environmental Engineering. 1996;122(2):141–148. doi: 10.1061/(ASCE)0733-9372(1996)122:2(141). - DOI
-
- Rzepecki LM, Waite JH. The Byssus of the Zebra Mussel, Dreissena polymorpha. I: Morphology and in situ Protein Processing During Maturation. Molecular Marine Biology and Biotechnology. 1993;2(5):255–266. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

