Simple fluorometric-based assay of antibiotic effectiveness for Acinetobacter baumannii biofilms
- PMID: 31004100
- PMCID: PMC6474882
- DOI: 10.1038/s41598-019-42353-0
Simple fluorometric-based assay of antibiotic effectiveness for Acinetobacter baumannii biofilms
Abstract
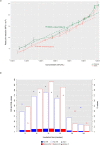
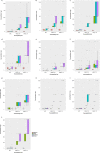
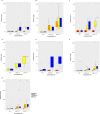
Despite strengthened antimicrobial therapy, biofilm infections of Acinetobacter baumannii are associated with poor prognosis and limited therapeutic options. Assessing antibiotics on planktonic bacteria can result in failure against biofilm infections. Currently, antibiotics to treat biofilm infections are administered empirically, usually without considering the susceptibility of the biofilm objectively before beginning treatment. For effective therapy to resolve biofilm infections it is essential to assess the efficacy of commonly used antibiotics against biofilms. Here, we offer a robust and simple assay to assess the efficacy of antibiotics against biofilms. In the present work, we carefully optimized the incubation time, detection range, and fluorescence reading mode for resazurin-based viability staining of biofilms in 96-well-plates and determined minimal biofilm eradication concentrations (MBECs) for A. baumannii isolates from patients with chronic infection. By applying this assay, we demonstrated that antibiotic response patterns varied uniquely within the biofilm formation of various clinical samples. MBEC-50 and 75 have significant discriminatory power over minimum inhibitory concentrations for planktonic suspensions to differentiate the overall efficiency of an antibiotic to eradicate a biofilm. The present assay is an ideal platform on which to assess the efficacy of antibiotics against biofilms in vitro to pave the way for more effective therapy.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

