Modelling changes in glutathione homeostasis as a function of quinone redox metabolism
- PMID: 31004119
- PMCID: PMC6474874
- DOI: 10.1038/s41598-019-42799-2
Modelling changes in glutathione homeostasis as a function of quinone redox metabolism
Abstract
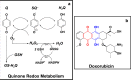
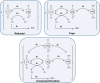
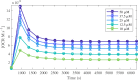
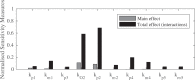
Redox cycling is an understated mechanism of toxicity associated with a plethora of xenobiotics, responsible for preventing the effective treatment of serious conditions such as malaria and cardiomyopathy. Quinone compounds are notorious redox cyclers, present in drugs such as doxorubicin, which is used to treat a host of human cancers. However, the therapeutic index of doxorubicin is undermined by dose-dependent cardiotoxicity, which may be a function of futile redox cycling. In this study, a doxorubicin-specific in silico quinone redox metabolism model is described. Doxorubicin-GSH adduct formation kinetics are thermodynamically estimated from its reduction potential, while the remainder of the model is parameterised using oxygen consumption rate data, indicative of hydroquinone auto-oxidation. The model is then combined with a comprehensive glutathione metabolism model, facilitating the simulation of quinone redox cycling, and adduct-induced GSH depletion. Simulations suggest that glutathione pools are most sensitive to exposure duration at pharmacologically and supra-pharmacologically relevant doxorubicin concentrations. The model provides an alternative method of investigating and quantifying redox cycling induced oxidative stress, circumventing the experimental difficulties of measuring and tracking radical species. This in silico framework provides a platform from which GSH depletion can be explored as a function of a compound's physicochemical properties.
Conflict of interest statement
The authors declare no competing interests.
Figures
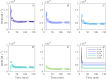
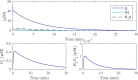
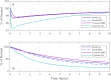
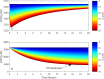
Similar articles
-
Molecular mechanisms of quinone cytotoxicity.Chem Biol Interact. 1991;80(1):1-41. doi: 10.1016/0009-2797(91)90029-7. Chem Biol Interact. 1991. PMID: 1913977 Review.
-
Myeloperoxidase-catalyzed metabolism of etoposide to its quinone and glutathione adduct forms in HL60 cells.Chem Res Toxicol. 2006 Jul;19(7):937-43. doi: 10.1021/tx0600595. Chem Res Toxicol. 2006. PMID: 16841962
-
Thiol oxidation coupled to DT-diaphorase-catalysed reduction of diaziquone. Reductive and oxidative pathways of diaziquone semiquinone modulated by glutathione and superoxide dismutase.Biochem J. 1992 Sep 1;286 ( Pt 2)(Pt 2):481-90. doi: 10.1042/bj2860481. Biochem J. 1992. PMID: 1530580 Free PMC article.
-
Hepatic low-level chemiluminescence during redox cycling of menadione and the menadione-glutathione conjugate: relation to glutathione and NAD(P)H:quinone reductase (DT-diaphorase) activity.Arch Biochem Biophys. 1983 Jul 15;224(2):568-78. doi: 10.1016/0003-9861(83)90244-8. Arch Biochem Biophys. 1983. PMID: 6191666
-
Nrf2/ARE-mediated antioxidant actions of pro-electrophilic drugs.Free Radic Biol Med. 2013 Dec;65:645-657. doi: 10.1016/j.freeradbiomed.2013.07.022. Epub 2013 Jul 25. Free Radic Biol Med. 2013. PMID: 23892355 Free PMC article. Review.
Cited by
-
Fruticuline A, a chemically-defined diterpene, exerts antineoplastic effects in vitro and in vivo by multiple mechanisms.Sci Rep. 2020 Oct 5;10(1):16477. doi: 10.1038/s41598-020-73432-2. Sci Rep. 2020. PMID: 33020521 Free PMC article.
-
Why Do Dietary Flavonoids Have a Promising Effect as Enhancers of Anthracyclines? Hydroxyl Substituents, Bioavailability and Biological Activity.Int J Mol Sci. 2022 Dec 26;24(1):391. doi: 10.3390/ijms24010391. Int J Mol Sci. 2022. PMID: 36613834 Free PMC article. Review.
-
Prenatal exposure to polycyclic aromatic hydrocarbons and gestational age at birth.Environ Int. 2022 Jun;164:107246. doi: 10.1016/j.envint.2022.107246. Epub 2022 Apr 15. Environ Int. 2022. PMID: 35453081 Free PMC article.
-
Identification of Modules With Similar Gene Regulation and Metabolic Functions Based on Co-expression Data.Front Mol Biosci. 2019 Dec 13;6:139. doi: 10.3389/fmolb.2019.00139. eCollection 2019. Front Mol Biosci. 2019. PMID: 31921888 Free PMC article.
-
Role of Glutathione in Cancer: From Mechanisms to Therapies.Biomolecules. 2020 Oct 9;10(10):1429. doi: 10.3390/biom10101429. Biomolecules. 2020. PMID: 33050144 Free PMC article. Review.
References
-
- Dudka J, et al. Intensification of Doxorubicin-Related Oxidative Stress in the Heart by Hypothyroidism Is Not Related to the Expression of Cytochrome P450 NADPH-Reductase and Inducible Nitric Oxide Synthase, As Well As Activity of Xanthine Oxidase. Oxid. Med. Cell. Longev. 2012;2012:139327. doi: 10.1155/2012/139327. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

