Examination of novel 4-aminoquinoline derivatives designed and synthesized by a hybrid pharmacophore approach to enhance their anticancer activities
- PMID: 31004122
- PMCID: PMC6474902
- DOI: 10.1038/s41598-019-42816-4
Examination of novel 4-aminoquinoline derivatives designed and synthesized by a hybrid pharmacophore approach to enhance their anticancer activities
Abstract
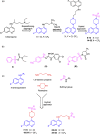
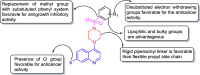
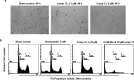
In an attempt to develop effective and potentially safe anticancer agents, thirty-six 4-aminoquinoline derived sulfonyl analogs were designed and synthesized using a hybrid pharmacophore approach. The cytotoxicity of these compounds was determined using three breast tumor cell lines (MDA-MB231, MDA-MB468 and MCF7) and two matching non-cancer breast epithelial cell lines (184B5 and MCF10A). Although most of the compounds were quite effective on the breast cancer cells, the compound 7-chloro-4-(4-(2,4-dinitrophenylsulfonyl)piperazin-1-yl)quinoline (13; VR23) emerged as potentially the most desirable one in this series of compounds. Data from the NCI-60 cancer panel screening show that compound 13 is effective on a wide range of different cancers. Importantly, compound 13 is needed up to 17.6-fold less doses to achieve the same IC50 against cancer than non-cancer cells (MDA-MB468 vs MCF10A), suggesting that it can potentially be less toxic to normal cells. Cancer cells formed multiple centrosomes in the presence of compound 13, resulting in the cell cycle arrest at prometa-meta phase. This abnormality leads to eventual cell demise with sub-G1 DNA content typically shown with apoptotic cells. In addition, compound 13 also causes an increase in lysosomal volume in cancer but not in non-cancer cells, which may contribute at least in part to its preferential cancer cell-killing. The cancer cell-killing effect of compound 13 is highly potentiated when combined with either bortezomib or monastrol.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Design and synthesis of 4-piperazinyl quinoline derived urea/thioureas for anti-breast cancer activity by a hybrid pharmacophore approach.J Enzyme Inhib Med Chem. 2019 Dec;34(1):620-630. doi: 10.1080/14756366.2019.1571055. J Enzyme Inhib Med Chem. 2019. PMID: 30727782 Free PMC article.
-
Design, synthesis and characterization of novel quinacrine analogs that preferentially kill cancer over non-cancer cells through the down-regulation of Bcl-2 and up-regulation of Bax and Bad.Eur J Med Chem. 2017 Sep 8;137:156-166. doi: 10.1016/j.ejmech.2017.05.052. Epub 2017 May 27. Eur J Med Chem. 2017. PMID: 28586716
-
Design and synthesis of anti-breast cancer agents from 4-piperazinylquinoline: a hybrid pharmacophore approach.Bioorg Med Chem. 2010 Feb 15;18(4):1563-72. doi: 10.1016/j.bmc.2010.01.001. Epub 2010 Jan 11. Bioorg Med Chem. 2010. PMID: 20106668
-
Biological Activity, Apoptotic Induction and Cell Cycle Arrest of New Hydrazonoyl Halides Derivatives.Anticancer Agents Med Chem. 2019;19(9):1141-1149. doi: 10.2174/1871520619666190306123658. Anticancer Agents Med Chem. 2019. PMID: 30843494
-
Hybrid pharmacophore design and synthesis of isatin-benzothiazole analogs for their anti-breast cancer activity.Bioorg Med Chem. 2009 Nov 1;17(21):7585-92. doi: 10.1016/j.bmc.2009.08.068. Epub 2009 Sep 15. Bioorg Med Chem. 2009. PMID: 19804979
Cited by
-
Concept of Hybrid Drugs and Recent Advancements in Anticancer Hybrids.Pharmaceuticals (Basel). 2022 Aug 28;15(9):1071. doi: 10.3390/ph15091071. Pharmaceuticals (Basel). 2022. PMID: 36145292 Free PMC article. Review.
-
Design, Synthesis and Anticancer Evaluation of Substituted Cinnamic Acid Bearing 2-Quinolone Hybrid Derivatives.Molecules. 2021 Aug 4;26(16):4724. doi: 10.3390/molecules26164724. Molecules. 2021. PMID: 34443308 Free PMC article.
-
Novel Oxadiazole-Quinoxalines as Hybrid Scaffolds with Antitumor Activity.Int J Mol Sci. 2025 Feb 8;26(4):1439. doi: 10.3390/ijms26041439. Int J Mol Sci. 2025. PMID: 40003907 Free PMC article.
-
Advances in antitumor research of CA-4 analogs carrying quinoline scaffold.Front Chem. 2022 Oct 28;10:1040333. doi: 10.3389/fchem.2022.1040333. eCollection 2022. Front Chem. 2022. PMID: 36385996 Free PMC article. Review.
-
Synthesis, Biocidal and Antibiofilm Activities of New Isatin-Quinoline Conjugates against Multidrug-Resistant Bacterial Pathogens along with Their In Silico Screening.Antibiotics (Basel). 2022 Oct 28;11(11):1507. doi: 10.3390/antibiotics11111507. Antibiotics (Basel). 2022. PMID: 36358162 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

