The Kansas University DHA Outcomes Study (KUDOS) clinical trial: long-term behavioral follow-up of the effects of prenatal DHA supplementation
- PMID: 31004139
- PMCID: PMC6499507
- DOI: 10.1093/ajcn/nqz018
The Kansas University DHA Outcomes Study (KUDOS) clinical trial: long-term behavioral follow-up of the effects of prenatal DHA supplementation
Abstract
Background: Docosahexaenoic acid (DHA) is a long-chain polyunsaturated fatty acid that has been linked to improved vision and cognition in postnatal feeding studies and has been consistently associated with reduction of early preterm birth in prenatal supplementation trials. This is a report of the first long-term follow-up of infants from mothers receiving prenatal DHA supplementation in a US cohort.
Objective: Our objective was to evaluate the efficacy of the prenatal supplementation on both global and granular longitudinal assessments of cognitive and behavioral development.
Methods: In a randomized double-blind clinical trial, mothers received either 600 mg/d of DHA or a placebo beginning at 14.5 weeks of gestation and capsules were provided until delivery. Children from those pregnancies were followed by cognitive and behavioral assessments administered from 10 mo through 6 y of age. From 301 mothers in the initial study, ∼200 infants completed the longitudinal schedule.
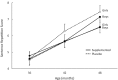
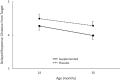
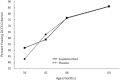
Results: Although this intervention had been shown to reduce high-risk pregnancies and improve visual attention in infants during the first year, only a few positive long-term effects of prenatal DHA supplementation emerged from analyses of this follow-up. Increases in maternal blood DHA during pregnancy were related to verbal and full scale intelligence quotient (IQ) scores at 5 and 6 y, but these effects disappeared after controlling for SES. Maternal blood DHA concentrations at delivery were unrelated to outcomes, although maternal DHA at enrollment was related to productive vocabulary at 18 mo.
Conclusions: Although prenatal DHA supplementation substantially reduced early preterm birth and improved visual attention in infancy in this sample, no consistent long-term benefits were observed into childhood. Increases in maternal blood DHA concentration in pregnancy were related to higher IQs but this effect was confounded with SES and disappeared when SES was statistically controlled. This trial was registered at http://www.clinicaltrials.gov as NCT00266825 and NCT02487771.
Keywords: behavior; clinical trial; development; docosahexaenoic acid; longitudinal; prenatal.
Copyright © American Society for Nutrition 2019.
Figures

Similar articles
-
Prenatal supplementation with DHA improves attention at 5 y of age: a randomized controlled trial.Am J Clin Nutr. 2016 Oct;104(4):1075-1082. doi: 10.3945/ajcn.114.101071. Epub 2016 Sep 7. Am J Clin Nutr. 2016. PMID: 27604770 Free PMC article. Clinical Trial.
-
Effect of Prenatal Docosahexaenoic Acid Supplementation on Blood Pressure in Children With Overweight Condition or Obesity: A Secondary Analysis of a Randomized Clinical Trial.JAMA Netw Open. 2019 Feb 1;2(2):e190088. doi: 10.1001/jamanetworkopen.2019.0088. JAMA Netw Open. 2019. PMID: 30794304 Free PMC article. Clinical Trial.
-
The effect of prenatal docosahexaenoic acid supplementation on infant outcomes in African American women living in low-income environments: A randomized, controlled trial.Psychoneuroendocrinology. 2016 Sep;71:170-5. doi: 10.1016/j.psyneuen.2016.05.023. Epub 2016 May 25. Psychoneuroendocrinology. 2016. PMID: 27290652 Free PMC article. Clinical Trial.
-
Omega-3 Fatty Acids and Maternal and Child Health: An Updated Systematic Review.Evid Rep Technol Assess (Full Rep). 2016 Oct;(224):1-826. doi: 10.23970/AHRQEPCERTA224. Evid Rep Technol Assess (Full Rep). 2016. PMID: 30307735
-
n-3 Fatty Acid Supplementation in Mothers, Preterm Infants, and Term Infants and Childhood Psychomotor and Visual Development: A Systematic Review and Meta-Analysis.J Nutr. 2018 Mar 1;148(3):409-418. doi: 10.1093/jn/nxx031. J Nutr. 2018. PMID: 29546296 Free PMC article.
Cited by
-
Science-based policy: targeted nutrition for all ages and the role of bioactives.Eur J Nutr. 2021 Oct;60(Suppl 1):1-17. doi: 10.1007/s00394-021-02662-5. Epub 2021 Aug 24. Eur J Nutr. 2021. PMID: 34427766 Free PMC article.
-
The Influence of Omega-3 Long-Chain Polyunsaturated Fatty Acid, Docosahexaenoic Acid, on Child Behavioral Functioning: A Review of Randomized Controlled Trials of DHA Supplementation in Pregnancy, the Neonatal Period and Infancy.Nutrients. 2021 Jan 28;13(2):415. doi: 10.3390/nu13020415. Nutrients. 2021. PMID: 33525526 Free PMC article. Review.
-
Nutrition and Brain Development.Curr Top Behav Neurosci. 2022;53:131-165. doi: 10.1007/7854_2021_244. Curr Top Behav Neurosci. 2022. PMID: 34622395
-
Omega-3 Fatty Acid Dietary Supplements Consumed During Pregnancy and Lactation and Child Neurodevelopment: A Systematic Review.J Nutr. 2021 Nov 2;151(11):3483-3494. doi: 10.1093/jn/nxab238. J Nutr. 2021. PMID: 34383914 Free PMC article.
-
Immune regulation of neurodevelopment at the mother-foetus interface: the case of autism.Clin Transl Immunology. 2020 Nov 13;9(11):e1211. doi: 10.1002/cti2.1211. eCollection 2020. Clin Transl Immunology. 2020. PMID: 33209302 Free PMC article. Review.
References
-
- Jensen CL, Heird WC, Anderson RE. Effect of maternal docosahexaenoic (DHA) supplementation on milk and infant plasma DHA. Pediatr Res. 1996;39(4):1857.
-
- Wainwright PE, Huang YS, Coscina DV, Levesque S, McCutcheon D. Brain-effects and behavioral-effects of dietary n-3 deficiency in mice: a 3-generational study. Dev Psychobiol. 1994;27(7):467–87. - PubMed
-
- Willatts P, Forsyth JS, DiModugno MK, Varma S, Colvin M. Effect of long-chain polyunsaturated fatty acids in infant formula on problem solving at 10 months of age. Lancet. 1998;352(9129):688–91. - PubMed
-
- Forsyth JS, Willatts P, Ross P, Mires GJ. Relationship of prenatal LCPUFA status to infant visual and cognitive function. J Reprod Infant Psyc. 2003;21(3):253.

