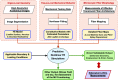
On the simulation of mitral valve function in health, disease, and treatment
- PMID: 31004145
- PMCID: PMC6611349
- DOI: 10.1115/1.4043552
On the simulation of mitral valve function in health, disease, and treatment
Abstract
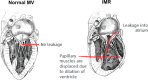
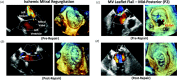
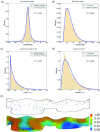
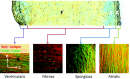
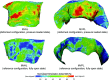
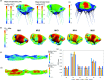
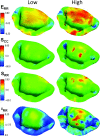
The mitral valve (MV) is the heart valve that regulates blood ?ow between the left atrium and left ventricle (LV). In situations where the MV fails to fully cover the left atrioventricular ori?ce during systole, the resulting regurgitation causes pulmonary congestion, leading to heart failure and/or stroke. The causes of MV insuf?ciency can be either primary (e.g. myxomatous degeneration) where the valvular tissue is organically diseased, or secondary (typically inducded by ischemic cardiomyopathy) termed ischemic mitral regurgitation (IMR), is brought on by adverse LV remodeling. IMR is present in up to 40% of patients and more than doubles the probability of cardiovascular morbidity after 3.5 years. There is now agreement that adjunctive procedures are required to treat IMR caused by lea?et tethering. However, there is no consensus regarding the best procedure. Multicenter registries and randomized trials would be necessary to prove which procedure is superior. Given the number of proposed procedures and the complexity and duration of such studies, it is highly unlikely that IMR procedure optimization will be achieved by prospective clinical trials. There is thus an urgent need for cell and tissue physiologically based quantitative assessments of MV function to better design surgical solutions and associated therapies. Novel computational approaches directed towards optimized surgical repair procedures can substantially reduce the need for such trial-and-error approaches. We present the details of our MV modeling techniques, with an emphasis on what is known and investigated at various length scales.
Figures











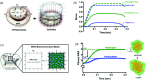
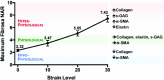
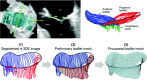
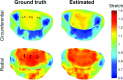
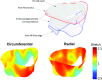
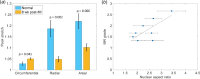

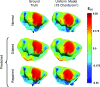
References
-
- Atluri, P. , Panlilio, C. M. , Liao, G. P. , Suarez, E. E. , McCormick, R. C. , Hiesinger, W. , Cohen, J. E. , Smith, M. J. , Patel, A. B. , Feng, W. , and Woo, Y. J. , 2008, “ Transmyocardial Revascularization to Enhance Myocardial Vasculogenesis and Hemodynamic Function,” J. Thorac. Cardiovasc. Surg., 135(2), pp. 283–291. 10.1016/j.jtcvs.2007.09.043 - DOI - PubMed
-
- Gillinov, A. M. , Wierup, P. N. , Blackstone, E. H. , Bishay, E. S. , Cosgrove, D. M. , White, J. , Lytle, B. W. , and McCarthy, P. M. , 2001, “ Is Repair Preferable to Replacement for Ischemic Mitral Regurgitation?,” J. Thorac. Cardiovasc. Surg., 122(6), pp. 1125–1141. 10.1067/mtc.2001.116557 - DOI - PubMed
-
- Acker, M. A. , Parides, M. K. , Perrault, L. P. , Moskowitz, A. J. , Gelijns, A. C. , Voisine, P. , Smith, P. K. , Hung, J. W. , Blackstone, E. H. , Puskas, J. D. , Argenziano, M. , Gammie, J. S. , Mack, M. , Ascheim, D. D. , Bagiella, E. , Moquete, E. G. , Ferguson, T. B. , Horvath, K. A. , Geller, N. L. , Miller, M. A. , Woo, Y. J. , D'Alessandro, D. A. , Ailawadi, G. , Dagenais, F. , Gardner, T. J. , O'Gara, P. T. , Michler, R. E. , and Kron, I. L. , 2014, “ Mitral-Valve Repair Versus Replacement for Severe Ischemic Mitral Regurgitation,” New Engl. J. Med., 370(1), pp. 23–32. 10.1056/NEJMoa1312808 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
