Applicability of the Mutation-Selection Balance Model to Population Genetics of Heterozygous Protein-Truncating Variants in Humans
- PMID: 31004148
- PMCID: PMC6738481
- DOI: 10.1093/molbev/msz092
Applicability of the Mutation-Selection Balance Model to Population Genetics of Heterozygous Protein-Truncating Variants in Humans
Abstract
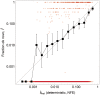
The fate of alleles in the human population is believed to be highly affected by the stochastic force of genetic drift. Estimation of the strength of natural selection in humans generally necessitates a careful modeling of drift including complex effects of the population history and structure. Protein-truncating variants (PTVs) are expected to evolve under strong purifying selection and to have a relatively high per-gene mutation rate. Thus, it is appealing to model the population genetics of PTVs under a simple deterministic mutation-selection balance, as has been proposed earlier (Cassa et al. 2017). Here, we investigated the limits of this approximation using both computer simulations and data-driven approaches. Our simulations rely on a model of demographic history estimated from 33,370 individual exomes of the Non-Finnish European subset of the ExAC data set (Lek et al. 2016). Additionally, we compared the African and European subset of the ExAC study and analyzed de novo PTVs. We show that the mutation-selection balance model is applicable to the majority of human genes, but not to genes under the weakest selection.
Keywords: genetic drift; protein-truncating variants; selection inference.
© The Author(s) 2019. Published by Oxford University Press on behalf of the Society for Molecular Biology and Evolution. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

