Protein Quality Control in the Endoplasmic Reticulum
- PMID: 31004255
- PMCID: PMC6589386
- DOI: 10.1007/s10930-019-09831-w
Protein Quality Control in the Endoplasmic Reticulum
Abstract
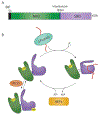
The site of protein folding and maturation for the majority of proteins that are secreted, localized to the plasma membrane or targeted to endomembrane compartments is the endoplasmic reticulum (ER). It is essential that proteins targeted to the ER are properly folded in order to carry out their function, as well as maintain protein homeostasis, as accumulation of misfolded proteins could lead to the formation of cytotoxic aggregates. Because protein folding is an error-prone process, the ER contains protein quality control networks that act to optimize proper folding and trafficking of client proteins. If a protein is unable to reach its native state, it is targeted for ER retention and subsequent degradation. The protein quality control networks of the ER that oversee this evaluation or interrogation process that decides the fate of maturing nascent chains is comprised of three general types of families: the classical chaperones, the carbohydrate-dependent system, and the thiol-dependent system. The cooperative action of these families promotes protein quality control and protein homeostasis in the ER. This review will describe the families of the ER protein quality control network and discuss the functions of individual members.
Keywords: Endoplasmic reticulum; Molecular chaperones; N-linked glycosylation; Oxidoreductases; Quality control; Secretory pathway.
Conflict of interest statement
Figures




References
-
- Blobel G, Sabatini DD (1971) Ribosome-membrane interaction in eukaryotic cells. Biomem 2:193–195
-
- Bulleid NJ, Freedman RB (1988) Defective co-translational formation of disulphide bonds in protein disulphide-isomerase-deficient microsomes. Nature 335:649–651 - PubMed
-
- Nicchitta CV, Blobel G (1993) Lumenal proteins of the mammalian endoplasmic reticulum are required to complete protein translocation. Cell 73:989–998 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

