Safety and Efficacy of 0.5% Carbomer 980 Gel for Treatment of Symptoms of Common Cold: Results of 2 Randomized Trials
- PMID: 31004286
- PMCID: PMC6544708
- DOI: 10.1007/s40268-019-0270-3
Safety and Efficacy of 0.5% Carbomer 980 Gel for Treatment of Symptoms of Common Cold: Results of 2 Randomized Trials
Erratum in
-
Correction to: Safety and Efficacy of 0.5% Carbomer 980 Gel for Treatment of Symptoms of Common Cold: Results of 2 Randomized Trials.Drugs R D. 2019 Sep;19(3):307. doi: 10.1007/s40268-019-0279-7. Drugs R D. 2019. PMID: 31327147 Free PMC article.
Abstract
Background: Two studies of intranasal 0.5% carbomer 980 gel were conducted evaluating nasal tolerability in healthy volunteers and safety and efficacy in adults with common cold symptoms.
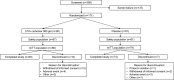
Methods: Study 1 randomly assigned healthy adults to 0.5% carbomer 980 gel (n = 20) or placebo (n = 10) administered intranasally four times daily for 7 days. Nasal examinations were conducted at baseline and daily throughout the study. The primary endpoint was local nasal tolerability. Study 2 randomly assigned adults with an investigator-confirmed diagnosis of symptomatic common cold to 0.5% carbomer 980 gel (n = 87) or placebo (n = 81), administered intranasally four times daily for 7 days (except for day 1, where subjects who received their first dose mid-day administered only three doses). The primary efficacy endpoint was the average nasal symptom score over days 1‒4 (ANSS1-4). Secondary efficacy endpoints included ANSS over days 1‒7 and average total symptom score (ATSS). Adverse events (AEs) were recorded throughout the study.
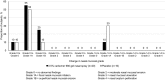
Results: In study 1, subjects assigned to 0.5% carbomer 980 gel had no mucosal grading higher than grade 1B (superficial nasal mucosal erosion) and low incidences of mucosal bleeding and crusting. In study 2, there were no statistically significant differences between treatments for any efficacy endpoints. Active treatment was well-tolerated; the most commonly reported AEs were headache, myalgia, and cough.
Conclusion: While 0.5% carbomer 980 gel nasal spray demonstrated good local nasal tolerability in healthy volunteers, the spray did not significantly impact the course of infection or resolution of cold symptoms in subjects with common cold.
Conflict of interest statement
Gilbert Shanga and Lara Dennie are employees of GlaxoSmithKline Consumer Healthcare.
Figures
References
-
- Hull JD, Barton IP, Torgersen J, McNeil CM. A survey of the experience and impact of acute upper respiratory tract infections on people in six countries in the 2011/2012 common cold and flu season. Open J Respir Dis. 2013;3:175–187.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical