Economic Evaluation of Sarilumab in the Treatment of Adult Patients with Moderately-to-Severely Active Rheumatoid Arthritis Who Have an Inadequate Response to Conventional Synthetic Disease-Modifying Antirheumatic Drugs
- PMID: 31004324
- PMCID: PMC6824456
- DOI: 10.1007/s12325-019-00946-1
Economic Evaluation of Sarilumab in the Treatment of Adult Patients with Moderately-to-Severely Active Rheumatoid Arthritis Who Have an Inadequate Response to Conventional Synthetic Disease-Modifying Antirheumatic Drugs
Abstract
Introduction: Assess the cost-effectiveness (US healthcare payer perspective) of sarilumab subcutaneous (SC) 200 mg + methotrexate versus conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) or targeted DMARD + methotrexate for moderate-to-severe rheumatoid arthritis (RA) in adults with inadequate response to methotrexate.
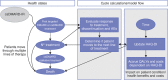
Methods: Microsimulation based on patient profiles from MOBILITY (NCT01061736) was conducted via a 6-month decision tree and lifetime Markov model with 6-monthly cycles. Treatment response at 6 months was informed by a network meta-analysis and based on American College of Rheumatology (ACR) response. Responders: patients with ACR20 response who continued with therapy; non-responders: ACR20 non-responders who transitioned to the subsequent treatment. Utilities and quality-adjusted life-years (QALYs) were estimated via mapping 6-month ACR20/50/70 response to relative change in Health Assessment Questionnaire Disability Index score (short term) and based on published algorithms (long term). Direct costs considered drugs (wholesale acquisition costs), administration and routine care.
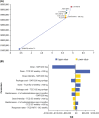
Results: Lifetime QALYs and costs for treatment sequences on the efficiency frontier were 3.43 and $115,019 for active csDMARD, 5.79 and $430,918 for sarilumab, and 5.94 and $524,832 for etanercept (all others dominated). Sarilumab was cost-effective versus tocilizumab and csDMARD (incremental cost-effectiveness ratios of $84,079/QALY and $134,286/QALY). Probabilistic sensitivity analysis suggested comparable costs and slightly improved health benefits for sarilumab versus tocilizumab, irrespective of threshold.
Conclusion: In patients with moderate-to-severe RA, sarilumab 200 mg SC every 2 weeks + methotrexate can be considered a cost-effective treatment option, with lower costs and greater health benefits than alternative treatment sequences (+ methotrexate) beginning with adalimumab, certolizumab, golimumab and tofacitinib and below commonly accepted cost-effectiveness thresholds against tocilizumab + methotrexate or csDMARD active treatment.
Funding: Sanofi and Regeneron Pharmaceuticals, Inc.
Keywords: Cost effectiveness; Disease-modifying anti-rheumatic; IL-6; Rheumatoid arthritis; Sarilumab.
Figures

References
-
- Shah A, St Clair W, et al. Rheumatoid arthritis. In: Kasper D, Fauci A, Hauser S, et al., editors. Harrison’s principles of internal medicine. 19. New York: McGraw-Hill Education; 2015.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

