Current advances in haploid stem cells
- PMID: 31004328
- PMCID: PMC6949308
- DOI: 10.1007/s13238-019-0625-0
Current advances in haploid stem cells
Abstract
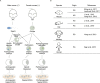
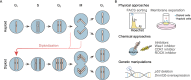
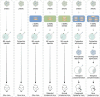
Diploidy is the typical genomic mode in all mammals. Haploid stem cells are artificial cell lines experimentally derived in vitro in the form of different types of stem cells, which combine the characteristics of haploidy with a broad developmental potential and open the possibility to uncover biological mysteries at a genomic scale. To date, a multitude of haploid stem cell types from mouse, rat, monkey and humans have been derived, as more are in development. They have been applied in high-throughput genetic screens and mammalian assisted reproduction. Here, we review the generation, unique properties and broad applications of these remarkable cells.
Keywords: androgenetic; diploidization; functional genomics; haploidy; imprinting; parthenogenetic; stem cells.
Figures

Similar articles
-
Genome wide functional genetics in haploid cells.FEBS Lett. 2014 Aug 1;588(15):2415-21. doi: 10.1016/j.febslet.2014.06.032. Epub 2014 Jun 17. FEBS Lett. 2014. PMID: 24950427 Review.
-
Haploidy in Humans: An Evolutionary and Developmental Perspective.Dev Cell. 2017 Jun 19;41(6):581-589. doi: 10.1016/j.devcel.2017.04.019. Dev Cell. 2017. PMID: 28633015 Review.
-
Haploid embryonic stem cells can be enriched and maintained by simple filtration.J Biol Chem. 2018 Apr 6;293(14):5230-5235. doi: 10.1074/jbc.RA118.002029. Epub 2018 Feb 15. J Biol Chem. 2018. PMID: 29449377 Free PMC article.
-
Durable pluripotency and haploidy in epiblast stem cells derived from haploid embryonic stem cells in vitro.J Mol Cell Biol. 2015 Aug;7(4):326-37. doi: 10.1093/jmcb/mjv044. Epub 2015 Jul 13. J Mol Cell Biol. 2015. PMID: 26169120
-
Medaka haploid embryonic stem cells.Methods Cell Biol. 2010;100:55-69. doi: 10.1016/B978-0-12-384892-5.00003-7. Methods Cell Biol. 2010. PMID: 21111214
Cited by
-
Genome Editing Using Cas9 Ribonucleoprotein Is Effective for Introducing PDGFRA Variant in Cultured Human Glioblastoma Cell Lines.Int J Mol Sci. 2022 Dec 28;24(1):500. doi: 10.3390/ijms24010500. Int J Mol Sci. 2022. PMID: 36613947 Free PMC article.
-
A Genetic Screen Identifies Etl4-Deficiency Capable of Stabilizing the Haploidy in Embryonic Stem Cells.Stem Cell Reports. 2021 Jan 12;16(1):29-38. doi: 10.1016/j.stemcr.2020.11.016. Stem Cell Reports. 2021. PMID: 33440180 Free PMC article.
-
Development and application of haploid embryonic stem cells.Stem Cell Res Ther. 2024 Apr 23;15(1):116. doi: 10.1186/s13287-024-03727-y. Stem Cell Res Ther. 2024. PMID: 38654389 Free PMC article. Review.
-
High-throughput screening in postimplantation haploid epiblast stem cells reveals Hs3st3b1 as a modulator for reprogramming.Stem Cells Transl Med. 2021 May;10(5):743-755. doi: 10.1002/sctm.20-0468. Epub 2021 Jan 29. Stem Cells Transl Med. 2021. PMID: 33511777 Free PMC article.
-
Male gamete copies to characterize genome inheritance and generate progenies.Sci Rep. 2025 May 4;15(1):15600. doi: 10.1038/s41598-025-99188-1. Sci Rep. 2025. PMID: 40320458 Free PMC article.
References
-
- Bai M, Wu Y, Li J. Generation and application of mammalian haploid embryonic stem cells. J Intern Med. 2016;280:236–245. - PubMed
-
- Beukeboom LW, Kamping A, Louter M, Pijnacker LP, Katju V, Ferree PM, Werren JH. Haploid females in the parasitic wasp Nasonia vitripennis. Science. 2007;315:206. - PubMed
-
- Brons IGM, Smithers LE, Trotter MWB, Rugg-Gunn P, Sun B, de Sousa Chuva, Lopes SM, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

