Young and middle-aged mouse breathing behavior during the light and dark cycles
- PMID: 31004390
- PMCID: PMC6474843
- DOI: 10.14814/phy2.14060
Young and middle-aged mouse breathing behavior during the light and dark cycles
Abstract
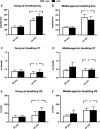
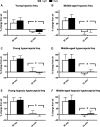
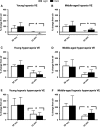
Unrestrained barometric plethysmography is a common method used for characterizing breathing patterns in small animals. One source of variation between unrestrained barometric plethysmography studies is the segment of baseline. Baseline may be analyzed as a predetermined time-point, or using tailored segments when each animal is visually calm. We compared a quiet, minimally active (no sniffing/grooming) breathing segment to a predetermined time-point at 1 h for baseline measurements in young and middle-aged mice during the dark and light cycles. Additionally, we evaluated the magnitude of change for gas challenges based on these two baseline segments. C57BL/6JEiJ x C3Sn.BliA-Pde6b+ /DnJ male mice underwent unrestrained barometric plethysmography with the following baselines used to determine breathing frequency, tidal volume (VT) and minute ventilation (VE): (1) 30-sec of quiet breathing and (2) a 10-min period from 50 to 60 min. Animals were also exposed to 10 min of hypoxic (10% O2 , balanced N2 ), hypercapnic (5% CO2 , balanced air) and hypoxic hypercapnic (10% O2 , 5% CO2 , balanced N2 ) gas. Both frequency and VE were higher during the predetermined 10-min baseline versus the 30-sec baseline, while VT was lower (P < 0.05). However, VE/VO2 was similar between the baseline time segments (P > 0.05) in an analysis of one cohort. During baseline, dark cycle testing had increased VT values versus those in the light (P < 0.05). For gas challenges, both frequency and VE showed higher percent change from the 30-sec baseline compared to the predetermined 10-min baseline (P < 0.05), while VT showed a greater change from the 10-min baseline (P < 0.05). Dark cycle hypoxic exposure resulted in larger percent change in breathing frequency versus the light cycle (P < 0.05). Overall, light and dark cycle pattern of breathing differences emerged along with differences between the 30-sec behavior observational method versus a predetermined time segment for baseline.
Keywords: Hypercapnia; hypoxia; hypoxic hypercapnia.
© 2019 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
The authors have no conflict of interest to disclose.
Figures


References
-
- Aschoff, J. , and Pohl H.. 1970. Rhythmic variations in energy metabolism. Feder. Proc. 29:1541–1552. - PubMed
-
- Basso, A. , Del Bello G., Piacenza F., Giacconi R., Costarelli L., and Malavolta M.. 2016. Circadian rhythms of body temperature and locomotor activity in aging BALB/c mice: early and late life span predictors. Biogerontology 17:703–714. - PubMed
-
- Bisgard, G. E. , and Neubauer J. A.. 1995. Peripheral and central effects of hypoxia. Regul. Breathing 79:617–668.
-
- Dauger, S. , Ferkdadji L., Saumon G., Vardon G., Peuchmaur M., Gaultier C., et al. 2003. Neonatal exposure to 65% oxygen durably impairs lung architecture and breathing pattern in adult mice. Chest 123:530–538. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

