Fulvestrant in the treatment of hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: A review
- PMID: 31004402
- PMCID: PMC6536994
- DOI: 10.1002/cam4.2095
Fulvestrant in the treatment of hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: A review
Abstract
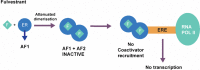
Nearly 75% of breast cancers are hormone receptor-positive (HR+) and human epidermal growth factor receptor type 2-negative (HER2-), making endocrine therapy the mainstay of treatment for HR+ and HER2- combination. Although endocrine therapy, such as therapy with fulvestrant, is widely used in the clinic, endocrine resistance (primary or secondary) is inevitable and poses a serious clinical concern. However, the therapeutic landscape of HR+/HER2- breast cancer is rapidly changing and evolving. In recent years, molecular insights into the genome of HR+/HER2- breast cancer have helped to identify promising targets, such as alterations in signaling pathways [phosphatidylinositide 3-kinase (PI3K/AKT/mammalian target of rapamycin (mTOR)], dysregulation of the cell cycle (CDK4/6), and identification of new ESR1 mutations. These insights have led to the development of newer targeted therapies, which aims at significantly improving survival in these patients. This review summarizes the role and rationale of fulvestrant when used as a monotherapy or in combination with targeted therapies in patients with HR+/HER2- advanced breast cancer. We also discuss other novel agents and potential future combination treatment options.
Keywords: HER2−; HR+; advanced breast cancer; fulvestrant; targeted therapies.
© 2019 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures
References
-
- Rugo HS, Rumble RB, Macrae E, et al. Endocrine therapy for hormone receptor‐positive metastatic breast cancer: American Society of Clinical Oncology guideline. J. Clin. Oncol. 2016;34:3069‐3103. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

