Optimizing Donor Cellular Dissociation and Subretinal Injection Parameters for Stem Cell-Based Treatments
- PMID: 31004408
- PMCID: PMC6646699
- DOI: 10.1002/sctm.18-0210
Optimizing Donor Cellular Dissociation and Subretinal Injection Parameters for Stem Cell-Based Treatments
Abstract
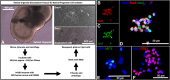
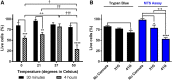
Subretinal delivery of stem cell-derived retinal cells as a strategy to treat retinal degenerative blindness holds great promise. Currently, two clinical trials are underway in which human fetal retinal progenitor cells (RPCs) are being delivered to patients by intravitreal or subretinal injection to preserve or restore vision, respectively. With the advent of the induced pluripotent stem cell (iPSC), and in turn three-dimensional derivation of retinal tissue, it is now possible to generate autologous RPCs for cell replacement. The purpose of this study was to evaluate the effect of commonly used cell isolation and surgical manipulation strategies on donor cell viability. iPSC-RPCs were subjected to various conditions, including different dissociation and isolation methods, injection cannula sizes, and preinjection storage temperatures and times. The effects of commonly used surgical techniques on both host and donor cell viability were evaluated in Yucatan mini-pigs (n = 61 eyes). We found a significant increase in cell viability when papain was used for RPC isolation. In addition, a significant decrease in cell viability was detected when using the 41G cannula compared with 31G and at storage times of 4 hours compared with 30 minutes. Although 96.4% of all eyes demonstrated spontaneous retinal reattachment following injection, retinal pigment epithelium (RPE) abnormalities were seen more frequently in eyes receiving injections via a 31G cannula; interestingly, eyes that received cell suspensions were relatively protected against such RPE changes. These findings indicate that optimization of donor cell isolation and delivery parameters should be considered when developing a subretinal cell replacement strategy. Stem Cells Translational Medicine 2019;8:797&809.
Keywords: Induced pluripotent stem cells; Inherited retinal dystrophy; Pig model; Retinal progenitor cells; Stem cell transplantation; Subretinal transplantation.
© 2019 The Authors. Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Conflict of interest statement
E.H.S. declared research funding from Oxford Biomedica; Sanofi; Regeneron. S.R.R. declared research funding from Spark Therapeutics and ProQR and is a consultant to Novartis. The other authors indicated no potential conflicts of interest.
Figures




Similar articles
-
Allogenic iPSC-derived RPE cell transplants induce immune response in pigs: a pilot study.Sci Rep. 2015 Jul 3;5:11791. doi: 10.1038/srep11791. Sci Rep. 2015. PMID: 26138532 Free PMC article.
-
Biocompatibility of Human Induced Pluripotent Stem Cell-Derived Retinal Progenitor Cell Grafts in Immunocompromised Rats.Cell Transplant. 2022 Jan-Dec;31:9636897221104451. doi: 10.1177/09636897221104451. Cell Transplant. 2022. PMID: 35758274 Free PMC article.
-
Transplantation of Human Embryonic Stem Cell-Derived Retinal Tissue in the Subretinal Space of the Cat Eye.Stem Cells Dev. 2019 Sep 1;28(17):1151-1166. doi: 10.1089/scd.2019.0090. Epub 2019 Jul 22. Stem Cells Dev. 2019. PMID: 31210100 Free PMC article.
-
Autologous stem cell therapy for inherited and acquired retinal disease.Regen Med. 2018 Jan;13(1):89-96. doi: 10.2217/rme-2017-0089. Epub 2018 Jan 23. Regen Med. 2018. PMID: 29360008 Free PMC article. Review.
-
Regenerative Medicine: Solution in Sight.Adv Exp Med Biol. 2016;854:543-8. doi: 10.1007/978-3-319-17121-0_72. Adv Exp Med Biol. 2016. PMID: 26427457 Review.
Cited by
-
Robot-assisted subretinal injection system: development and preliminary verification.BMC Ophthalmol. 2022 Dec 12;22(1):484. doi: 10.1186/s12886-022-02720-4. BMC Ophthalmol. 2022. PMID: 36510151 Free PMC article.
-
12-month outcomes after voretigene neparvovec gene therapy in paediatric patients with RPE65-mediated inherited retinal dystrophy.Br J Ophthalmol. 2025 Jan 28;109(2):281-285. doi: 10.1136/bjo-2024-326221. Br J Ophthalmol. 2025. PMID: 39578019 Free PMC article.
-
Could internal limiting membrane peeling before Voretigen neparvovec-ryzl subretinal injection prevent focal chorioretinal atrophy?Heliyon. 2024 Jan 26;10(3):e25154. doi: 10.1016/j.heliyon.2024.e25154. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38322949 Free PMC article.
-
Transplanted human induced pluripotent stem cells- derived retinal ganglion cells embed within mouse retinas and are electrophysiologically functional.iScience. 2022 Oct 7;25(11):105308. doi: 10.1016/j.isci.2022.105308. eCollection 2022 Nov 18. iScience. 2022. PMID: 36388952 Free PMC article.
-
Subretinal Transplantation of Human Embryonic Stem Cell-Derived Retinal Tissue in a Feline Large Animal Model.J Vis Exp. 2021 Aug 5;(174):10.3791/61683. doi: 10.3791/61683. J Vis Exp. 2021. PMID: 34424232 Free PMC article.
References
-
- Klassen HJ, Ng TF, Kurimoto Y et al. Multipotent retinal progenitors express developmental markers, differentiate into retinal neurons, and preserve light‐mediated behavior. Invest Ophthalmol Vis Sci 2004;45:4167–4173. - PubMed
-
- MacLaren RE, Pearson RA, MacNeil A et al. Retinal repair by transplantation of photoreceptor precursors. Nature 2006;444:203–207. - PubMed
-
- Bartsch U, Oriyakhel W, Kenna PF et al. Retinal cells integrate into the outer nuclear layer and differentiate into mature photoreceptors after subretinal transplantation into adult mice. Exp Eye Res 2008;86:691–700. - PubMed
-
- Santos‐Ferreira T, Völkner M, Borsch O et al. Stem cell‐derived photoreceptor transplants differentially integrate into mouse models of cone‐rod dystrophy. Invest Ophthalmol Vis Sci 2016;57:3509–3520. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

