Staphylococcus aureus Colonization of the Human Nose and Interaction with Other Microbiome Members
- PMID: 31004422
- PMCID: PMC11590430
- DOI: 10.1128/microbiolspec.GPP3-0029-2018
Staphylococcus aureus Colonization of the Human Nose and Interaction with Other Microbiome Members
Abstract
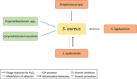
Staphylococcus aureus is usually regarded as a bacterial pathogen due to its ability to cause multiple types of invasive infections. Nevertheless, S. aureus colonizes about 30% of the human population asymptomatically in the nares, either transiently or persistently, and can therefore be regarded a human commensal as well, although carriage increases the risk of infection. Whereas many facets of the infection processes have been studied intensively, little is known about the commensal lifestyle of S. aureus. Recent studies highlight the major role of the composition of the highly variable nasal microbiota in promoting or inhibiting S. aureus colonization. Competition for limited nutrients, trace elements, and epithelial attachment sites, different susceptibilities to host defense molecules and the production of antimicrobial molecules by bacterial competitors may determine whether nasal bacteria outcompete each other. This chapter summarizes our knowledge about mechanisms that are used by S. aureus for efficient nasal colonization and strategies used by other nasal bacteria to interfere with its colonization. An improved understanding of naturally evolved mechanisms might enable us to develop new strategies for pathogen eradication.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

