Repressive role of stabilized hypoxia inducible factor 1α expression on transforming growth factor β-induced extracellular matrix production in lung cancer cells
- PMID: 31004547
- PMCID: PMC6549927
- DOI: 10.1111/cas.14027
Repressive role of stabilized hypoxia inducible factor 1α expression on transforming growth factor β-induced extracellular matrix production in lung cancer cells
Abstract
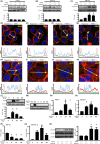
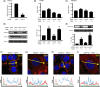
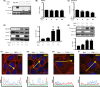
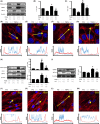
Activation of transforming growth factor β (TGF-β) combined with persistent hypoxia often affects the tumor microenvironment. Disruption of cadherin/catenin complexes induced by these stimulations yields aberrant extracellular matrix (ECM) production, characteristics of epithelial-mesenchymal transition (EMT). Hypoxia-inducible factors (HIF), the hallmark of the response to hypoxia, play differential roles during development of diseases. Recent studies show that localization of cadherin/catenin complexes at the cell membrane might be tightly regulated by protein phosphatase activity. We aimed to investigate the role of stabilized HIF-1α expression by protein phosphatase activity on dissociation of the E-cadherin/β-catenin complex and aberrant ECM expression in lung cancer cells under stimulation by TGF-β. By using lung cancer cells treated with HIF-1α stabilizers or carrying doxycycline-dependent HIF-1α deletion or point mutants, we investigated the role of stabilized HIF-1α expression on TGF-β-induced EMT in lung cancer cells. Furthermore, the underlying mechanisms were determined by inhibition of protein phosphatase activity. Persistent stimulation by TGF-β and hypoxia induced EMT phenotypes in H358 cells in which stabilized HIF-1α expression was inhibited. Stabilized HIF-1α protein expression inhibited the TGF-β-stimulated appearance of EMT phenotypes across cell types and species, independent of de novo vascular endothelial growth factor A (VEGFA) expression. Inhibition of protein phosphatase 2A activity abrogated the HIF-1α-induced repression of the TGF-β-stimulated appearance of EMT phenotypes. This is the first study to show a direct role of stabilized HIF-1α expression on inhibition of TGF-β-induced EMT phenotypes in lung cancer cells, in part, through protein phosphatase activity.
Keywords: HIF-1α; TGF-β; epithelial-mesenchymal transition; hypoxia; lung cancer.
© 2019 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures



Similar articles
-
Hypoxia-inducible factor prolyl-hydroxylase-2 mediates transforming growth factor beta 1-induced epithelial-mesenchymal transition in renal tubular cells.Biochim Biophys Acta. 2013 Jun;1833(6):1454-62. doi: 10.1016/j.bbamcr.2013.02.029. Epub 2013 Mar 1. Biochim Biophys Acta. 2013. PMID: 23466866 Free PMC article.
-
Hypoxia-inducible factor 1α-induced epithelial-mesenchymal transition of endometrial epithelial cells may contribute to the development of endometriosis.Hum Reprod. 2016 Jun;31(6):1327-38. doi: 10.1093/humrep/dew081. Epub 2016 Apr 19. Hum Reprod. 2016. PMID: 27094478
-
Inhibition of hypoxia inducible factor-1α downregulates the expression of epithelial to mesenchymal transition early marker proteins without undermining cell survival in hypoxic lens epithelial cells.Mol Vis. 2015 Sep 1;21:1024-35. eCollection 2015. Mol Vis. 2015. PMID: 26392741 Free PMC article.
-
HIF-1α-Mediated Disruption of Cellular Junctions: The Impact of Hypoxia on the Tumor Microenvironment and Invasion.Int J Mol Sci. 2025 May 26;26(11):5101. doi: 10.3390/ijms26115101. Int J Mol Sci. 2025. PMID: 40507913 Free PMC article. Review.
-
Hypoxia-regulated target genes implicated in tumor metastasis.J Biomed Sci. 2012 Dec 14;19(1):102. doi: 10.1186/1423-0127-19-102. J Biomed Sci. 2012. PMID: 23241400 Free PMC article. Review.
Cited by
-
Unveiling the link between chronic inflammation and cancer.Metabol Open. 2025 Jan 9;25:100347. doi: 10.1016/j.metop.2025.100347. eCollection 2025 Mar. Metabol Open. 2025. PMID: 39876904 Free PMC article. Review.
-
A Prognostic Risk Score Based on Hypoxia-, Immunity-, and Epithelialto-Mesenchymal Transition-Related Genes for the Prognosis and Immunotherapy Response of Lung Adenocarcinoma.Front Cell Dev Biol. 2022 Jan 24;9:758777. doi: 10.3389/fcell.2021.758777. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35141229 Free PMC article.
-
The Synergistic Cooperation between TGF-β and Hypoxia in Cancer and Fibrosis.Biomolecules. 2022 Apr 25;12(5):635. doi: 10.3390/biom12050635. Biomolecules. 2022. PMID: 35625561 Free PMC article. Review.
-
HIF-1α facilitates glioma proliferation and invasion by activating pyroptosis signaling axis.Chin Neurosurg J. 2024 May 11;10(1):14. doi: 10.1186/s41016-024-00366-3. Chin Neurosurg J. 2024. PMID: 38734702 Free PMC article.
-
Pharmacological Activities and Mechanisms of Hirudin and Its Derivatives - A Review.Front Pharmacol. 2021 Apr 16;12:660757. doi: 10.3389/fphar.2021.660757. eCollection 2021. Front Pharmacol. 2021. PMID: 33935784 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

