Mechanisms that regulate morphogenesis of a highly branched neuron in C. elegans
- PMID: 31004567
- PMCID: PMC7755292
- DOI: 10.1016/j.ydbio.2019.04.002
Mechanisms that regulate morphogenesis of a highly branched neuron in C. elegans
Abstract
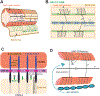
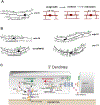
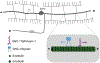
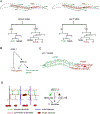
The shape of an individual neuron is linked to its function with axons sending signals to other cells and dendrites receiving them. Although much is known of the mechanisms for axonal outgrowth, the striking complexity of dendritic architecture has hindered efforts to uncover pathways that direct dendritic branching. Here we review the results of an experimental strategy that exploits the power of genetic analysis and live cell imaging of the PVD sensory neuron in C. elegans to reveal key molecular drivers of dendrite morphogenesis.
Copyright © 2019. Published by Elsevier Inc.
Figures


Similar articles
-
Conserved RNA-binding proteins required for dendrite morphogenesis in Caenorhabditis elegans sensory neurons.G3 (Bethesda). 2015 Feb 10;5(4):639-53. doi: 10.1534/g3.115.017327. G3 (Bethesda). 2015. PMID: 25673135 Free PMC article.
-
Dendrite morphogenesis in Caenorhabditis elegans.Genetics. 2024 Jun 5;227(2):iyae056. doi: 10.1093/genetics/iyae056. Genetics. 2024. PMID: 38785371 Free PMC article. Review.
-
GRDN-1/Girdin regulates dendrite morphogenesis and cilium position in two specialized sensory neuron types in C. elegans.Dev Biol. 2021 Apr;472:38-51. doi: 10.1016/j.ydbio.2020.12.022. Epub 2021 Jan 16. Dev Biol. 2021. PMID: 33460640 Free PMC article.
-
Separate transcriptionally regulated pathways specify distinct classes of sister dendrites in a nociceptive neuron.Dev Biol. 2017 Dec 15;432(2):248-257. doi: 10.1016/j.ydbio.2017.10.009. Epub 2017 Oct 13. Dev Biol. 2017. PMID: 29031632 Free PMC article.
-
Beyond being innervated: the epidermis actively shapes sensory dendritic patterning.Open Biol. 2019 Mar 29;9(3):180257. doi: 10.1098/rsob.180257. Open Biol. 2019. PMID: 30914004 Free PMC article. Review.
Cited by
-
Cortical anchoring of the microtubule cytoskeleton is essential for neuron polarity.Elife. 2020 Apr 15;9:e55111. doi: 10.7554/eLife.55111. Elife. 2020. PMID: 32293562 Free PMC article.
-
Neuron tracing and quantitative analyses of dendritic architecture reveal symmetrical three-way-junctions and phenotypes of git-1 in C. elegans.PLoS Comput Biol. 2021 Jul 19;17(7):e1009185. doi: 10.1371/journal.pcbi.1009185. eCollection 2021 Jul. PLoS Comput Biol. 2021. PMID: 34280180 Free PMC article.
-
PTRN-1 (CAMSAP) and NOCA-2 (NINEIN) are required for microtubule polarity in Caenorhabditis elegans dendrites.PLoS Biol. 2022 Nov 17;20(11):e3001855. doi: 10.1371/journal.pbio.3001855. eCollection 2022 Nov. PLoS Biol. 2022. PMID: 36395330 Free PMC article.
-
A role for the Erk MAPK pathway in modulating SAX-7/L1CAM-dependent locomotion in Caenorhabditis elegans.Genetics. 2022 Feb 4;220(2):iyab215. doi: 10.1093/genetics/iyab215. Genetics. 2022. PMID: 34849872 Free PMC article.
-
Optimization of RNAi efficiency in PVD neuron of C. elegans.PLoS One. 2024 Mar 18;19(3):e0298766. doi: 10.1371/journal.pone.0298766. eCollection 2024. PLoS One. 2024. PMID: 38498505 Free PMC article.
References
-
- Abraira VE, Kuehn ED, Chirila AM, Springel MW, Toliver AA, Zimmerman AL, Orefice LL, Boyle KA, Bai L, Song BJ, Bashista KA, O’Neill TG, Zhuo J, Tsan C, Hoynoski J, Rutlin M, Kus L, Niederkofler V, Watanabe M, Dymecki SM, Nelson SB, Heintz N, Hughes DI, Ginty DD, 2017. The cellular and synaptic architecture of the mechanosensory dorsal horn. Cell 168, 295–310. 10.1016/j.cell.2016.12.010 e19. - DOI - PMC - PubMed
-
- Bashaw GJ, Kidd T, Murray D, Pawson T, Goodman CS, 2000. Repulsive axon guidance: abelson and Enabled play opposing roles downstream of the roundabout receptor. Cell 101, 703–715. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

