Development of a novel multiplex electrochemiluminescent-based immunoassay to aid enterotoxigenic Escherichia coli vaccine development and evaluations
- PMID: 31004579
- PMCID: PMC6538825
- DOI: 10.1016/j.jim.2019.04.003
Development of a novel multiplex electrochemiluminescent-based immunoassay to aid enterotoxigenic Escherichia coli vaccine development and evaluations
Abstract
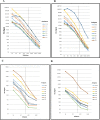
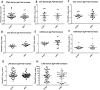
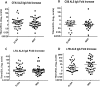
Enterotoxigenic Escherichia coli (ETEC) is a leading cause of bacterial diarrhea both among children in low and middle income countries and in travelers to these regions. Although there are several approaches to develop an effective vaccine for ETEC, no licensed vaccines are currently available. The most advanced ETEC vaccine candidates include multiple colonization factors along with the heat labile toxin B subunit. In the absence of known correlates of protection, and to understand the mechanism of protection, monitoring immune responses to a majority of the vaccine associated antigens using various types of samples is needed. Unfortunately, a traditional ELISA is time consuming, labor intensive and requires substantial amounts of antigens and sample volumes. To address these constraints, we developed and validated a novel high throughput electrochemiluminescent (ECL) - based multiplex immunoassay using Meso Scale Discovery (MSD) platform for analyzing immune responses to ETEC antigens. The ETEC multiplex ECL assay is an 8-plex assay which includes the ETEC colonization factor antigens (CFA/I, CS1, CS2, CS3, CS5 and CS6) along with the two subunits of heat labile toxin (LTA and LTB). Our data suggested that a single dilution of sample provides a quantifiable result for a wide range of sample titers. To compare ETEC multiplex ECL with ELISA, we carried out assays using the same antigens with the two immunoassay platforms using a common sample set of serum and ALS (antibodies in lymphocyte supernatant) specimens. The MSD platform achieved excellent correlations with ELISA for the antigens tested, consistently detecting comparable antibody levels in the samples. The ETEC multiplex ECL can serve as a fundamental platform in evaluating performances of candidate ETEC vaccines in future field trials.
Keywords: ELISA; ETEC; Immune responses; Multiplex ECL; Vaccine.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Intradermally Administered Enterotoxigenic Escherichia coli Vaccine Candidate MecVax Induces Functional Serum Immunoglobulin G Antibodies against Seven Adhesins (CFA/I and CS1 through CS6) and Both Toxins (STa and LT).Appl Environ Microbiol. 2022 Feb 22;88(4):e0213921. doi: 10.1128/AEM.02139-21. Epub 2021 Dec 22. Appl Environ Microbiol. 2022. PMID: 34936832 Free PMC article.
-
Development and preclinical evaluation of safety and immunogenicity of an oral ETEC vaccine containing inactivated E. coli bacteria overexpressing colonization factors CFA/I, CS3, CS5 and CS6 combined with a hybrid LT/CT B subunit antigen, administered alone and together with dmLT adjuvant.Vaccine. 2013 May 7;31(20):2457-64. doi: 10.1016/j.vaccine.2013.03.027. Epub 2013 Mar 27. Vaccine. 2013. PMID: 23541621
-
Significance of Enterotoxigenic Escherichia coli (ETEC) Heat-Labile Toxin (LT) Enzymatic Subunit Epitopes in LT Enterotoxicity and Immunogenicity.Appl Environ Microbiol. 2018 Jul 17;84(15):e00849-18. doi: 10.1128/AEM.00849-18. Print 2018 Aug 1. Appl Environ Microbiol. 2018. PMID: 29802193 Free PMC article.
-
Current Progress in Developing Subunit Vaccines against Enterotoxigenic Escherichia coli-Associated Diarrhea.Clin Vaccine Immunol. 2015 Sep;22(9):983-91. doi: 10.1128/CVI.00224-15. Epub 2015 Jul 1. Clin Vaccine Immunol. 2015. PMID: 26135975 Free PMC article. Review.
-
Review on pathogenicity mechanism of enterotoxigenic Escherichia coli and vaccines against it.Microb Pathog. 2018 Apr;117:162-169. doi: 10.1016/j.micpath.2018.02.032. Epub 2018 Feb 21. Microb Pathog. 2018. PMID: 29474827 Review.
Cited by
-
Linking inherent O-Linked Protein Glycosylation of YghJ to Increased Antigen Potential.Front Cell Infect Microbiol. 2021 Aug 19;11:705468. doi: 10.3389/fcimb.2021.705468. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34490144 Free PMC article.
-
Multiplexed Immunosensors and Immunoarrays.Anal Chem. 2020 Jan 7;92(1):345-362. doi: 10.1021/acs.analchem.9b05080. Epub 2019 Dec 2. Anal Chem. 2020. PMID: 31726821 Free PMC article. Review. No abstract available.
-
E. coli Enterotoxin LtB Enhances Vaccine-Induced Anti-H. pylori Protection by Promoting Leukocyte Migration into Gastric Mucus via Inflammatory Lesions.Cells. 2019 Aug 27;8(9):982. doi: 10.3390/cells8090982. Cells. 2019. PMID: 31461854 Free PMC article.
References
-
- Adhikari R.P., Haudenschild C., Sterba P.M., Sahandi S., Enterlein S., Holtsberg F.W., Aman M.J. Development of a novel multiplex electrochemiluminescent-based immunoassay for quantification of human serum IgG against 10 Staphylococcus aureus toxins. J. Immunol. Methods. 2016;430:33–42. - PubMed
-
- Bourgeois A.L., Wierzba T.F., Walker R.I. Status of vaccine research and development for enterotoxigenic Escherichia coli. Vaccine. 2016;34:2880–2886. - PubMed
-
- Carbonell-Estrany X., Simões E.A., Dagan R., Hall C.B., Harris B., Hultquist M., Connor E.M., Losonsky G.A., Motavizumab Study Group Motavizumab for prophylaxis of respiratory syncytial virus in high-risk children: a noninferiority trial. Pediatrics. 2010;125(1):e35–e51. - PubMed
-
- Chakraborty S., Harro C., DeNearing B., Bream J., Bauers N., Dally L., Flores J., Van de Verg L., Sack D.A., Walker R. Evaluation of the safety, tolerability, and immunogenicity of an oral, inactivated whole-cell Shigella flexneri 2a vaccine in healthy adult subjects. Clin. Vaccine Immunol. 2016;23(4):315–325. (Apr 4, Print 2016 Apr., PMID:26865592) - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

