A microparticle approach for non-viral gene delivery within 3D human mesenchymal stromal cell aggregates
- PMID: 31004846
- PMCID: PMC6888862
- DOI: 10.1016/j.actbio.2019.04.038
A microparticle approach for non-viral gene delivery within 3D human mesenchymal stromal cell aggregates
Abstract
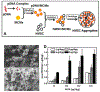
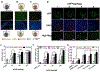
Three-dimensional (3D) multicellular aggregates, in comparison to two-dimensional monolayer culture, can provide tissue culture models that better recapitulate the abundant cell-cell and cell-matrix interactions found in vivo. In addition, aggregates are potentially useful building blocks for tissue engineering. However, control over the interior aggregate microenvironment is challenging due to inherent barriers for diffusion of biological mediators (e.g. growth factors) throughout the multicellular aggregates. Previous studies have shown that incorporation of biomaterials into multicellular aggregates can support cell survival and control differentiation of stem cell aggregates by delivering morphogens from within the 3D construct. In this study, we developed a highly efficient microparticle-based gene delivery approach to uniformly transfect human mesenchymal stromal cells (hMSC) within multicellular aggregates and cell sheets. We hypothesized that release of plasmid DNA (pDNA) lipoplexes from mineral-coated microparticles (MCMs) within 3D hMSC constructs would improve transfection in comparison to standard free pDNA lipoplex delivery in the media. Our approach increased transfection efficiency 5-fold over delivery of free pDNA lipoplexes in the media and resulted in homogenous distribution of transfected cells throughout the 3D constructs. Additionally, we found that MCMs improved hMSC transfection by specifically increasing macropinocytosis-mediated uptake of pDNA. Finally, we showed up to a three-fold increase of bone morphogenetic protein-2 (BMP-2) expression and enhanced calcium deposition within 3D hMSC constructs following MCM-mediated delivery of a BMP-2 encoding plasmid and culture in osteogenic medium. The technology described here provides a valuable tool for achieving efficient and homogenous transfection of 3D cell constructs and is therefore of particular value in tissue engineering and regenerative medicine applications. STATEMENT OF SIGNIFICANCE: This original research describes a materials-based approach, whereby use of mineral-coated microparticles improves the efficiency of non-viral gene delivery in three-dimensional human mesenchymal stromal cell constructs. Specifically, it demonstrates the use of mineral-coated microparticles to enable highly efficient transfection of human mesenchymal stromal cells in large, 3D culture formats. The manuscript also identifies specific endocytosis pathways that interact with the mineral coating to afford the improved transfection efficiency, as well as demonstrates the utility of this approach toward improving differentiation of large cell constructs. We feel that this manuscript will impact the current understanding and near-term development of materials for non-viral gene delivery in broad tissue engineering and biofabrication applications, and therefore be of interest to a diverse biomaterials audience.
Copyright © 2019. Published by Elsevier Ltd.
Conflict of interest statement
Disclosures and competing financial interests
W. Murphy is a founder and stockholder for Stem Pharm, Inc. and Tissue Regeneration Systems, Inc.
Figures


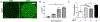
Similar articles
-
Physical and mechanical cues affecting biomaterial-mediated plasmid DNA delivery: insights into non-viral delivery systems.J Genet Eng Biotechnol. 2021 Jun 17;19(1):90. doi: 10.1186/s43141-021-00194-3. J Genet Eng Biotechnol. 2021. PMID: 34142237 Free PMC article. Review.
-
Controlled Dual Growth Factor Delivery From Microparticles Incorporated Within Human Bone Marrow-Derived Mesenchymal Stem Cell Aggregates for Enhanced Bone Tissue Engineering via Endochondral Ossification.Stem Cells Transl Med. 2016 Feb;5(2):206-17. doi: 10.5966/sctm.2015-0115. Epub 2015 Dec 23. Stem Cells Transl Med. 2016. PMID: 26702127 Free PMC article.
-
Dual non-viral gene delivery from microparticles within 3D high-density stem cell constructs for enhanced bone tissue engineering.Biomaterials. 2018 Apr;161:240-255. doi: 10.1016/j.biomaterials.2018.01.006. Epub 2018 Jan 3. Biomaterials. 2018. PMID: 29421560 Free PMC article.
-
Functionalization of microparticles with mineral coatings enhances non-viral transfection of primary human cells.Sci Rep. 2017 Oct 27;7(1):14211. doi: 10.1038/s41598-017-14153-x. Sci Rep. 2017. PMID: 29079806 Free PMC article.
-
Microfluidic technologies to engineer mesenchymal stem cell aggregates-applications and benefits.Biophys Rev. 2020 Feb;12(1):123-133. doi: 10.1007/s12551-020-00613-8. Epub 2020 Jan 17. Biophys Rev. 2020. PMID: 31953794 Free PMC article. Review.
Cited by
-
Light-controlled scaffold- and serum-free hard palatal-derived mesenchymal stem cell aggregates for bone regeneration.Bioeng Transl Med. 2022 May 13;8(1):e10334. doi: 10.1002/btm2.10334. eCollection 2023 Jan. Bioeng Transl Med. 2022. PMID: 36684075 Free PMC article.
-
Non-viral gene delivery to human mesenchymal stem cells: a practical guide towards cell engineering.J Biol Eng. 2023 Jul 25;17(1):49. doi: 10.1186/s13036-023-00363-7. J Biol Eng. 2023. PMID: 37491322 Free PMC article. Review.
-
Strategies to capitalize on cell spheroid therapeutic potential for tissue repair and disease modeling.NPJ Regen Med. 2022 Dec 9;7(1):70. doi: 10.1038/s41536-022-00266-z. NPJ Regen Med. 2022. PMID: 36494368 Free PMC article. Review.
-
Physical and mechanical cues affecting biomaterial-mediated plasmid DNA delivery: insights into non-viral delivery systems.J Genet Eng Biotechnol. 2021 Jun 17;19(1):90. doi: 10.1186/s43141-021-00194-3. J Genet Eng Biotechnol. 2021. PMID: 34142237 Free PMC article. Review.
-
Engineered biomaterials to guide spheroid formation, function, and fabrication into 3D tissue constructs.Acta Biomater. 2023 Jul 15;165:4-18. doi: 10.1016/j.actbio.2022.09.052. Epub 2022 Sep 24. Acta Biomater. 2023. PMID: 36167240 Free PMC article. Review.
References
-
- Mooney DJ, Vandenburgh H, Cell Delivery Mechanisms for Tissue Repair, Cell Stem Cell. 2 (2008) 205–213. - PubMed
-
- Brindley DA, Davie NL, Sahlman WA, Bonfiglio GA, Culme-Seymour EJ, Reeve BC, Mason C, Promising Growth and Investment in the Cell Therapy Industry during the First Quarter of 2012, Cell Stem Cell. 10 (2012) 492–496. - PubMed
-
- Li W-J, Laurencin CT, Caterson EJ, Tuan RS, Ko FK, Electrospun nanofibrous structure: a novel scaffold for tissue engineering., J. Biomed. Mater. Res 60 (2002) 613–21. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

