Downstream platelet adhesion and activation under highly elevated upstream shear forces
- PMID: 31004847
- PMCID: PMC6525641
- DOI: 10.1016/j.actbio.2019.04.028
Downstream platelet adhesion and activation under highly elevated upstream shear forces
Abstract
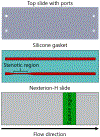
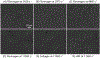
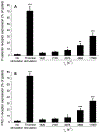
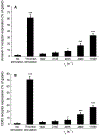
Elevated shear force caused by an anastomotic stenosis is a common complication at the blood vessel-vascular implant interface. Although elevated shear forces were found to cause platelet aggregation around a stenotic region, transient platelet exposure to elevated shear forces and subsequent downstream events occurring under lower shear force were not extensively studied. We hypothesize that effects of elevated shear forces on pre-activation of platelets for downstream adhesion and activation are relevant in understanding the increased thrombotic risk associated with blood-contacting devices. We designed a microfluidic flow system to mimic the hemodynamic environment of vasculature with an upstream anastomotic stenosis with five wall shear strain rates ranging from 1620 s-1 to 11560 s-1. Under shear flow conditions, transient exposure of whole blood to elevated shear forces resulted in higher downstream platelet adhesion onto three different immobilized platelet agonists: fibrinogen, collagen, or von Willebrand factor. Platelet expression of four activation markers (P-selectin, GPIIb/IIIa, lysosomal glycoprotein, and phosphatidylserine) significantly increased after transient exposure to higher upstream wall shear strain rates of 2975-11560 s-1. A significant lysis was observed when platelets were primed by upstream wall shear strain rate of 11560 s-1. These experimental results could be helpful to understand how altered hemodynamics around an anastomotic stenosis promotes thrombus formation downstream. STATEMENT OF SIGNIFICANCE: Studying the downstream response of platelets following transient exposure to an upstream agonist is important because of significant clinical implications to the implantation of vascular devices. Due to intimal fibrous hyperplasia, vascular biomaterials such as synthetic small-diameter vascular grafts sometimes become stenotic (narrow), leading to transient platelet exposure to elevated shear forces. In this study, a microfluidic flow system was developed to mimic a stenosed vascular graft and to investigate how highly elevated, transient upstream shear forces, typically found in severe stenosis, results in the pre-activation of platelets for downstream adhesion and activation. The findings of the present study have implications for optimizing the design of blood-contacting biomaterials in order to minimize thrombotic risk associated with transiently elevated shear forces. The findings also provide additional insights into the mechanisms of thrombus formation at the post-stenotic regions of vascular implants.
Keywords: Anastomotic stenosis; Flow cytometry; Microfluidics; Platelet adhesion and activation; Platelet lysis.
Copyright © 2019 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of Interest
The authors declare no conflict of interest.
Figures


Similar articles
-
Effects of upstream shear forces on priming of platelets for downstream adhesion and activation.Acta Biomater. 2018 Jun;73:228-235. doi: 10.1016/j.actbio.2018.04.002. Epub 2018 Apr 11. Acta Biomater. 2018. PMID: 29654993 Free PMC article.
-
Effects of elapsed time on downstream platelet adhesion following transient exposure to elevated upstream shear forces.Colloids Surf B Biointerfaces. 2020 Sep;193:111118. doi: 10.1016/j.colsurfb.2020.111118. Epub 2020 May 12. Colloids Surf B Biointerfaces. 2020. PMID: 32450503
-
Effect of pathological high shear exposure time on platelet activation and aggregation.Clin Hemorheol Microcirc. 2023;84(2):125-139. doi: 10.3233/CH-221567. Clin Hemorheol Microcirc. 2023. PMID: 37066902
-
[Platelet--vessel wall interactions].Therapie. 2006 Sep-Oct;61(5):371-8. doi: 10.2515/therapie:2006068. Therapie. 2006. PMID: 17243265 Review. French.
-
VWF attributes--impact on thrombus formation.Thromb Res. 2008;122 Suppl 4:S9-13. doi: 10.1016/S0049-3848(08)70028-8. Thromb Res. 2008. PMID: 18929523 Review.
Cited by
-
Tumor Cell Capture Using Platelet-Based and Platelet-Mimicking Modified Human Serum Albumin Submicron Particles.Int J Mol Sci. 2022 Nov 18;23(22):14277. doi: 10.3390/ijms232214277. Int J Mol Sci. 2022. PMID: 36430755 Free PMC article.
-
Effect of upstream priming on transient downstream platelet-substrate interactions.Colloids Surf B Biointerfaces. 2021 Oct;206:111925. doi: 10.1016/j.colsurfb.2021.111925. Epub 2021 Jun 21. Colloids Surf B Biointerfaces. 2021. PMID: 34175742 Free PMC article.
-
Emerging Elastic Micro-Nano Materials for Diagnosis and Treatment of Thrombosis.Research (Wash D C). 2025 Feb 28;8:0614. doi: 10.34133/research.0614. eCollection 2025. Research (Wash D C). 2025. PMID: 40028043 Free PMC article. Review.
-
Type 2 Diabetes Mellitus, Platelet Activation and Alzheimer's Disease: A Possible Connection.Clin Neuropsychiatry. 2022 Dec;19(6):370-378. doi: 10.36131/cnfioritieditore20220604. Clin Neuropsychiatry. 2022. PMID: 36627944 Free PMC article.
-
GPVI-mediated thrombus stabilization of shear-induced platelet aggregates in a microfluidic stenosis.Biophys J. 2025 Jan 7;124(1):158-171. doi: 10.1016/j.bpj.2024.11.018. Epub 2024 Nov 22. Biophys J. 2025. PMID: 39573878
References
-
- Swedberg SH, Brown BG, Sigley R, Wight TN, Gordon D, Nicholls SC, Intimal fibromuscular hyperplasia at the venous anastomosis of PTFE grafts in hemodialysis patients: clinical, immunocytochemical, light and electron microscopic assessment, Circulation 80 (1989) 1726–1736. - PubMed
-
- Wilson YG, Davies AH, Currie IC, Morgan M, Vein graft stenosis: incidence and intervention, Eur. J. Vasc. Endovasc. Surg 11 (1996) 164–169. - PubMed
-
- Zhao R, Kameneva MV, Antaki JF, Investigation of platelet margination phenomena at elevated shear stress, Biorheology 44 (2007) 161–177. - PubMed
-
- Sugimoto M, Tsuji S, Kuwahara M, Matsui H, Shear-dependent functions of the interaction between soluble von Willebrand factor and platelet glycoprotein Ib in mural thrombus formation on a collagen surface, J. Hematol 69 (1999) 48–53. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

