Will morphing boron-based inhibitors beat the β-lactamases?
- PMID: 31004962
- PMCID: PMC6591701
- DOI: 10.1016/j.cbpa.2019.03.001
Will morphing boron-based inhibitors beat the β-lactamases?
Abstract
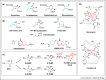
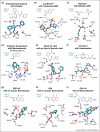
The β-lactams remain the most important antibacterials, but their use is increasingly compromised by resistance, importantly by β-lactamases. Although β-lactam and non-β-lactam inhibitors forming stable acyl-enzyme complexes with nucleophilic serine β-lactamases (SBLs) are widely used, these are increasingly susceptible to evolved SBLs and do not inhibit metallo-β-lactamases (MBLs). Boronic acids and boronate esters, especially cyclic ones, can potently inhibit both SBLs and MBLs. Vaborbactam, a monocyclic boronate, is approved for clinical use, but its β-lactamase coverage is limited. Bicyclic boronates rapidly react with SBLs and MBLs forming stable enzyme-inhibitor complexes that mimic the common anionic high-energy tetrahedral intermediates in SBL/MBL catalysis, as revealed by crystallography. The ability of boronic acids to 'morph' between sp2 and sp3 hybridisation states may help enable potent inhibition. There is limited structure-activity relationship information on the (bi)cyclic boronate inhibitors compared to β-lactams, hence scope for creativity towards new boron-based β-lactamase inhibitors/antibacterials.
Copyright © 2019 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


Similar articles
-
Studies on the inhibition of AmpC and other β-lactamases by cyclic boronates.Biochim Biophys Acta Gen Subj. 2019 Apr;1863(4):742-748. doi: 10.1016/j.bbagen.2019.02.004. Epub 2019 Feb 7. Biochim Biophys Acta Gen Subj. 2019. PMID: 30738906
-
Cyclic Boronates Inhibit All Classes of β-Lactamases.Antimicrob Agents Chemother. 2017 Mar 24;61(4):e02260-16. doi: 10.1128/AAC.02260-16. Print 2017 Apr. Antimicrob Agents Chemother. 2017. PMID: 28115348 Free PMC article.
-
Bicyclic Boronate VNRX-5133 Inhibits Metallo- and Serine-β-Lactamases.J Med Chem. 2019 Sep 26;62(18):8544-8556. doi: 10.1021/acs.jmedchem.9b00911. Epub 2019 Sep 16. J Med Chem. 2019. PMID: 31454231 Free PMC article.
-
Recent advances in β-lactamase inhibitor chemotypes and inhibition modes.Eur J Med Chem. 2022 Nov 15;242:114677. doi: 10.1016/j.ejmech.2022.114677. Epub 2022 Aug 13. Eur J Med Chem. 2022. PMID: 35988449 Review.
-
Metallo-β-lactamase-mediated antimicrobial resistance and progress in inhibitor discovery.Trends Microbiol. 2023 Jul;31(7):735-748. doi: 10.1016/j.tim.2023.01.013. Epub 2023 Feb 27. Trends Microbiol. 2023. PMID: 36858862 Review.
Cited by
-
Structural Basis of Metallo-β-lactamase Inhibition by N-Sulfamoylpyrrole-2-carboxylates.ACS Infect Dis. 2021 Jun 11;7(6):1809-1817. doi: 10.1021/acsinfecdis.1c00104. Epub 2021 May 18. ACS Infect Dis. 2021. PMID: 34003651 Free PMC article.
-
The Role of the Ω-Loop in Regulation of the Catalytic Activity of TEM-Type β-Lactamases.Biomolecules. 2019 Dec 11;9(12):854. doi: 10.3390/biom9120854. Biomolecules. 2019. PMID: 31835662 Free PMC article. Review.
-
Metallo-β-Lactamase Inhibitors Inspired on Snapshots from the Catalytic Mechanism.Biomolecules. 2020 Jun 3;10(6):854. doi: 10.3390/biom10060854. Biomolecules. 2020. PMID: 32503337 Free PMC article. Review.
-
Boronic Acids as Prospective Inhibitors of Metallo-β-Lactamases: Efficient Chemical Reaction in the Enzymatic Active Site Revealed by Molecular Modeling.Molecules. 2021 Apr 2;26(7):2026. doi: 10.3390/molecules26072026. Molecules. 2021. PMID: 33918209 Free PMC article.
-
Assessment of Activity and Resistance Mechanisms to Cefepime in Combination with the Novel β-Lactamase Inhibitors Zidebactam, Taniborbactam, and Enmetazobactam against a Multicenter Collection of Carbapenemase-Producing Enterobacterales.Antimicrob Agents Chemother. 2022 Feb 15;66(2):e0167621. doi: 10.1128/AAC.01676-21. Epub 2021 Nov 22. Antimicrob Agents Chemother. 2022. PMID: 34807754 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

