Imaging features of triple-negative breast cancers according to androgen receptor status
- PMID: 31005169
- PMCID: PMC6481318
- DOI: 10.1016/j.ejrad.2019.03.017
Imaging features of triple-negative breast cancers according to androgen receptor status
Abstract
Objective: Different molecular subtypes of triple-negative breast cancer (TNBC) have previously been identified through analysis of gene expression profiles. The luminal androgen receptor (LAR) subtype has been shown to have a lower rate of pathologic complete response to neoadjuvant chemotherapy than other TNBC subtypes. The purpose of this study was to determine if the imaging features of TNBCs differ by AR (androgen receptor) status, which is a surrogate immunohistochemical (IHC) marker for the chemoresistant LAR subtype of TNBC.
Materials and methods: This sub-study was part of a clinical trial in patients with stage I-III TNBC who were prospectively monitored for response while receiving neoadjuvant systemic therapy (NAST) at a single comprehensive cancer center. This interim imaging analysis included 144 patients with known AR status measured by IHC. AR-positive (AR+) tumors were defined as those in which at least 10% of tumor cells had positive nuclear AR staining. Two experienced, fellowship-trained breast radiologists who were blinded to the IHC results retrospectively reviewed and reached consensus on all imaging studies for the index lesion (i.e., mammogram, ultrasound, and breast magnetic resonance imaging). The index lesion for each patient was reviewed and described according to the fifth edition of the Breast Imaging Reporting and Data System lexicon. Logistic regression modeling was used to identify imaging features predictive of AR status. p ≤ 0.05 was considered statistically significant.
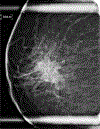
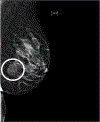
Results: Univariate logistic regression models for AR status showed that AR+ TNBC was significantly associated with heterogeneously dense breast composition on mammography (p = 0.02), mass with calcifications (p = 0.05), irregular mass shape on mammography (p = 0.03), and irregular mass shape on sonography (p = 0.003). Multivariate logistic regression models for AR status showed that AR+ TNBC was significantly associated with heterogeneously dense breast composition on mammography (p = 0.01), high mass density on mammography (p = 0.003), and irregular mass shape on sonography (p = 0.0004).
Conclusion: The imaging features of TNBCs differ by AR status. Multimodality breast imaging may help identify the LAR subtype of TNBC, which has been shown to be a subtype that is relatively resistant to neoadjuvant chemotherapy.
Keywords: Androgen receptor; MRI; Mammography; Triple-negative breast cancer; Ultrasound.
Copyright © 2019 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Interests
We wish to draw the attention of the Editor to the following facts which may be considered as potential conflicts of interest and to significant financial contributions to this work.
Rosalind P. Candelaria, MD – Nothing to disclose.
Beatriz E. Adrada, MD – Nothing to disclose.
Wei Wei, MS – Nothing to disclose.
Lumarie Santiago, MD – Nothing to disclose.
Deanna L. Lane, MD – Nothing to disclose.
Monica L. Huang, MD – Nothing to disclose.
Elsa M. Arribas, MD – Nothing to disclose.
Gaiane M. Rauch, MD, PhD – Nothing to disclose.
Michael Z. Gilcrease, MD, PhD – Nothing to disclose.
Lei Huo, MD, PhD – Nothing to disclose.
W. Fraser Symmans, MD – Delphi Diagnostics: intellectual property and founder shares; IONIS Pharmaceuticals: stock; Merck: advisory board honorarium; Almac Diagnostics: advisory board honorarium.
Alastair M. Thompson, MD – Pfizer: honorarium.
Bora Lim, MD – Pfizer: research funding
Naoto T. Ueno, MD, PhD – Pfizer: research funding; Epic Sciences: research funding
Stacy L. Moulder, MD – Oncothyreon: research funding and advisor; Seattle Genetics: research funding and advisor; Roche: research funding and advisor; Genentech: research funding and advisor; Novartis: research funding, advisor, and honorarium; Pfizer: research funding and advisor; EMD Serono: research funding and advisor; Bayer: advisor; Merck: advisor.
Wei Tse Yang, MD – Wolters Kluewer: consultant; GE Healthcare: consultant; Seno Medical Instruments: advisory committee member; Elsevier: royalties.
Declarations of interest:
• Rosalind P. Candelaria, MD – Nothing to disclose.
• Beatriz E. Adrada, MD – Nothing to disclose.
• Wei Wei, MS – Nothing to disclose.
• Lumarie Santiago, MD – Nothing to disclose.
• Deanna L. Lane, MD – Nothing to disclose.
• Monica L. Huang, MD – Nothing to disclose.
• Elsa M. Arribas, MD – Nothing to disclose.
• Gaiane M. Rauch, MD, PhD – Nothing to disclose.
• Michael Z. Gilcrease, MD, PhD – Nothing to disclose.
• Lei Huo, MD, PhD – Nothing to disclose.
• W. Fraser Symmans, MD – Delphi Diagnostics: intellectual property and founder shares; IONIS Pharmaceuticals: stock; Merck: advisory board honorarium; Almac Diagnostics: advisory board honorarium.
• Alastair M. Thompson, MD – Pfizer: honorarium.
• Bora Lim, MD – Pfizer: research funding.
• Naoto T. Ueno, MD, PhD – Pfizer: research funding; Epic Sciences: research funding.
• Stacy L. Moulder, MD – Oncothyreon: research funding and advisor; Seattle Genetics: research funding and advisor; Roche: research funding and advisor; Genentech: research funding and advisor; Novartis: research funding, advisor, and honorarium; Pfizer: research funding and advisor; EMD Serono: research funding and advisor; Bayer: advisor; Merck: advisor.
• Wei Tse Yang, MD – Wolters Kluewer: consultant; GE Healthcare: consultant; Seno Medical Instruments: advisory committee member; Elsevier: royalties.
Figures




Similar articles
-
Heterogeneity of triple-negative breast cancer: mammographic, US, and MR imaging features according to androgen receptor expression.Eur Radiol. 2015 Feb;25(2):419-27. doi: 10.1007/s00330-014-3419-z. Epub 2014 Sep 16. Eur Radiol. 2015. PMID: 25224727
-
Immunohistochemical co-expression status of cytokeratin 5/6, androgen receptor, and p53 as prognostic factors of adjuvant chemotherapy for triple negative breast cancer.Med Mol Morphol. 2016 Mar;49(1):11-21. doi: 10.1007/s00795-015-0109-0. Epub 2015 May 26. Med Mol Morphol. 2016. PMID: 26009308
-
Clinical Imaging of the Heterogeneous Group of Triple-negative Breast Cancer.Anticancer Res. 2020 Apr;40(4):2125-2131. doi: 10.21873/anticanres.14171. Anticancer Res. 2020. PMID: 32234905
-
Role of the androgen receptor in triple-negative breast cancer.Clin Adv Hematol Oncol. 2016 Mar;14(3):186-93. Clin Adv Hematol Oncol. 2016. PMID: 27058032 Free PMC article. Review.
-
Androgen receptor in triple negative breast cancer: A potential target for the targetless subtype.Cancer Treat Rev. 2018 Jul;68:102-110. doi: 10.1016/j.ctrv.2018.06.005. Epub 2018 Jun 11. Cancer Treat Rev. 2018. PMID: 29940524 Review.
Cited by
-
Molecular Classification, Treatment, and Genetic Biomarkers in Triple-Negative Breast Cancer: A Review.Technol Cancer Res Treat. 2023 Jan-Dec;22:15330338221145246. doi: 10.1177/15330338221145246. Technol Cancer Res Treat. 2023. PMID: 36601658 Free PMC article. Review.
-
Clinical Implications of Androgen-Positive Triple-Negative Breast Cancer.Cancers (Basel). 2021 Apr 1;13(7):1642. doi: 10.3390/cancers13071642. Cancers (Basel). 2021. PMID: 33915941 Free PMC article. Review.
-
Anti-Androgenic Therapies Targeting the Luminal Androgen Receptor of a Typical Triple-Negative Breast Cancer.Cancers (Basel). 2022 Dec 30;15(1):233. doi: 10.3390/cancers15010233. Cancers (Basel). 2022. PMID: 36612226 Free PMC article. Review.
-
Androgen receptor function and targeted therapeutics across breast cancer subtypes.Breast Cancer Res. 2022 Nov 14;24(1):79. doi: 10.1186/s13058-022-01574-4. Breast Cancer Res. 2022. PMID: 36376977 Free PMC article. Review.
-
Malignant Apocrine Lesions of the Breast: Multimodality Imaging Findings and Biologic Features.J Breast Cancer. 2022 Dec;25(6):513-521. doi: 10.4048/jbc.2022.25.e46. Epub 2022 Nov 14. J Breast Cancer. 2022. PMID: 36479602 Free PMC article.
References
-
- Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MD, Nielsen TO, Moorman PG, Earp HS, Millikan RC, Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 295 (2006) 2492–2502. - PubMed
-
- Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA, Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13(15 Pt 1) (2007) 4429–4434. - PubMed
-
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365(9472) (2005) 1687–1717. - PubMed
-
- Haffty BG, Yang Q, Reiss M, Kearney T, Higgins SA, Weidhaas J, Harris L, Hait W, Toppmeyer D, Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol 24(36) (2006) 5652–5657. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

